FAMily ties
Less than three months ago, FAM177A1 Research Foundation hosted its first research roundtable. Drug repurposing and biomarker discovery projects are now in flight. Gene therapy will be on deck soon.
In collaboration with
Jill Hawkins is not just the cofounder of Charlotte and Cooper. She’s also the leader of a community barreling toward the frontier of science. Not by choice but by necessity. Unlike any club you’d want to join or could leave at any time, most people in Jill’s community received an automatic lifetime membership because they have a loved one affected by homozygous loss-of-function variants in a mystery gene called FAM177A1.
FAM stands for “family with sequence similarity.” Overlooked genes receive the prefix FAM when no one knows what they do. Lost strangers in a strange land, the only clue to their identity is that FAM genes resemble another gene or group of genes in the genome. Hence the unique alphanumeric suffix. But it’s as informative and actionable as receiving a notification from 23andMe saying you have a new relative living on the other side of the world with whom you share some percentage of your DNA.
FAM genes are orphans among orphans. Yet even in numbers as small as two, there is strength and hope. FAM families — FAMilies — don’t have a choice when it comes to advancing research as fast as humanly possible.
The ones who do have a choice in all this are the scientists. FAMilies need scientists to answer the fundamental questions. What does this mystery gene FAM177A1 actually do? How do we fix a mercurial and cruel disease caused when this random gene on chromosome 14 is broken?
Scientists must be actively recruited to the community. It’s an often thankless but vital job that falls on pioneer parents and community founders like Jill to enlighten, charm, and if necessary cajole researchers to join the FAM177A1 Research Foundation mission to create clinically actionable knowledge where it didn’t exist before.
As described in the FAM177A1 CureMap v1.0, foundational disease modeling work is needed to get started because so little is empirically known about FAM177A1 function. When an ultra-rare disease community starts to nucleate, there aren’t enough diagnosed patients to support traditional natural history studies. The 1-to-N medicine approach offers a solution: riches in niches. It only takes a few FAMilies to pool resources in order to kickstart drug repurposing using a FAM177A1 patient avatar and biomarker discovery using FAM177A1 patients as living laboratories.
Yet in order to do drug repurposing, you need a suitable patient avatar. FAM1771A can be modeled in mice, zebrafish, flies and human cells.
We’ll discuss the FAM177A1 mouse modeling project at the end of this post. A manuscript describing a FAM177A1 knockout zebrafish model has been submitted for publication. Suffice it to say there isn’t an obvious disease phenotype in fish that is amenable to high-throughput screening. What about human cells? FAM177A1-knockout cells don’t present with a Golgi defect unless stressed with brefeldin A. That would be amenable for an image-based high-content screen but what if the Golgi defect isn’t the whole story? Cost considerations aside, what if rescuing the pharmacologically-induced Golgi defect doesn’t actually address the root cause of disease?
Turns out the fly is a middle ground. Working with Dr Clement Chow’s lab at the University of Utah, we explored the feasibility of a fly model of FAM177A1 deficiency. After exploring many options for a screenotype, the most robust and consistent proved to be male lethality. Although no parent ever wants to hear the word lethality uttered aloud, in the contexts of disease modeling and drug repurposing it has several advantages. Unlike an image-based readout in single cells, a fly is a whole animal for starters. Rescuing lethality is a lot more impressive than rescuing Golgi morphology in a cell, especially a phenotype that must be provoked by an exogenous stressor.
FAM177A1-deficient male flies fail to eclose, which is Drosophila-speak for almost fully formed adults either die in the pupal case, or die while trying to crawl out of the pupal case. If we find one or more compounds that result in the development of healthy adult male flies, we’re in business. The Chow lab started screening the Prestwick collection this month. We expect the screen to be completed by the end of the year.
You only need a single brave pioneer to embark on a journey of biomarker discovery. Once a trail is blazed, others will follow. Yet conventional wisdom would have ultra-rare disease communities focus on the diagnostic odyssey so as to increase the size of the known patient universe. After all, strength in numbers. At the same time, beware of vanity metrics. There is no magic threshold at which, for example, a natural history study becomes suddenly possible. Not to pick on natural history studies. The 1-to-N logic applies to any research endeavor.
Dr David Fajgenbaum — most recently the cofounder of Every Cure — proved that it truly only takes one pioneer to go from zero to one. Of course, it helps when patient, scientist and doctor are all rolled into one, as in David’s case. He chronicled his journey in his riveting book Chasing My Cure.
In parallel to the fly-powered drug repurposing project, the FAM177A1 Research Foundation is working with the company SomaLogic on a proteomics biomarker discovery project. Shamelessly taking a page from the Fajgenbaum playbook! Patient avatars have their place but there’s not substitute for human beings living with the disease.
To save on costs and ensure operational success, the Foundation is collaborating with CombinedBrain on sample collection. CombinedBrain is organizing a nationwide mobile phlebotomy roadshow that is scheduled to arrive in the Pacific Northwest in about six weeks. As fate would have it, three genetically unrelated and geographically separated FAMilies have two affected children who have at least one unaffected sibling or half-sibling. That allows for a case-control study design with familial structure. In other words, if we see a common set of proteins that are elevated or depleted only in FAM kiddos, we’re in business for a second time.
The potential for convergence between this biomarker discovery project and the drug repurposing is high. For example, a fly drug repurposing hit that inhibits a protein whose human homolog is increased in FAM177A1 patients compared to controls, or vice versa.
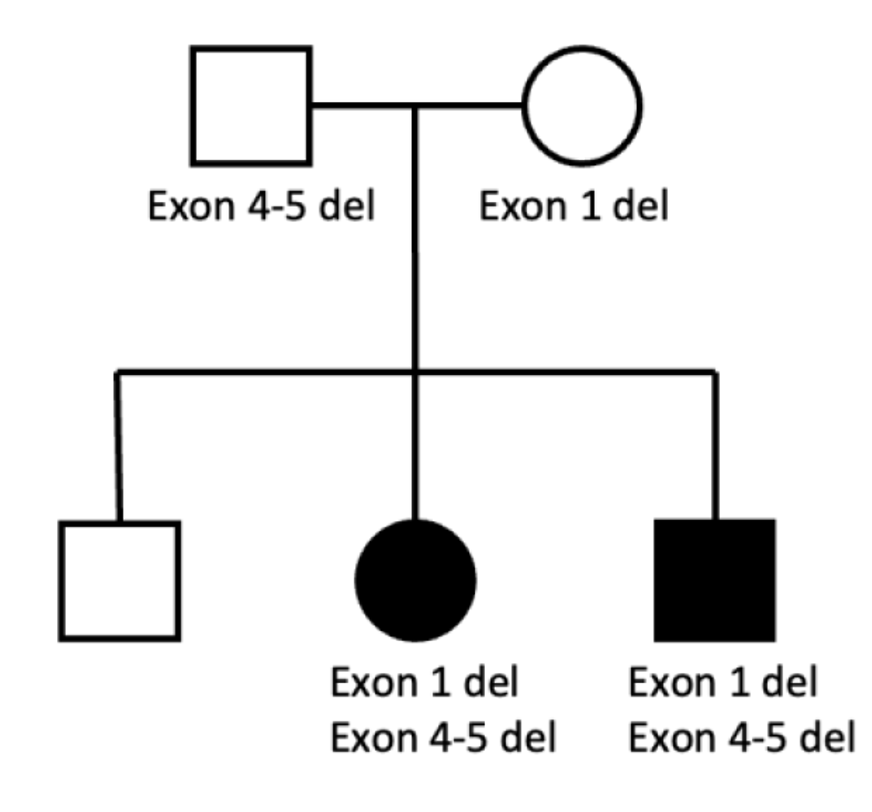
From a gene replacement perspective, FAM177A1 is an ideal candidate on paper. The genetics show that FAM177A1 is caused by homozygous loss-of-function variants, including deletion alleles. The prevalence of deletions even in the small number of diagnosed FAM kiddos is strong evidence that little to no residual FAM177A1 protein function is expected, further justifying a whole-gene replacement strategy.
Jill is putting all the pieces in place in preparation for a AAV gene therapy program, starting with a FAM177A1 mouse model. Dr Matthew Simon at Jackson Lab (JAX) is leading the effort to create a FAM177A1 knockout mouse. Initial phenotypic assessments are expected to begin in a few months depending on how breeding goes.
As an alum of the Ultragenyx Rare Entrepreneur bootcamp, Jill has stayed in touch with the members of the gene therapy team at Ultragenyx. In the weeks ahead, introductory conversations are planned with academic leaders in gene therapy to make sure there’s solid consensus around FAM177A1 as a gene therapy payload.
Stay tuned!