KCNC1 drug repurposing screen
Eliana's V434L gain-of-function variant was expressed in Chinese Hamster Ovary cells and the Broad Repurposing Hub library was screened for channel inhibitors. One of the top hits is a nutraceutical.
In collaboration with
Let’s start by centering this story around our pioneer patient and her family. Eliana has a de novo pathogenic variant, V434L, in her KCNC1 gene, which encodes a potassium channel called Kv3.1 that is predominantly expressed in inhibitory GABAergic interneurons and cerebellar neurons. A genetic lightning strike to the KCNC1 gene can present as myoclonic epilepsy and ataxia due to potassium channel (MEAK), developmental epileptic encephalopathy (DEE), or intellectual disability with hypotonia but no seizures. It all comes down to where lightning strikes.
A cryo-EM model of Kv3.1 is the featured image above. Four Kv3.1 subunits intertwine to form a pore through which the purple potassium ion flows. Imagine that you are facing down the barrel of the channel. Highlighted in red, V434 resides near the center of the channel and at the interface between helices. This interface is key for channel function, as these dynamic helices need to slide past each other for the channel to switch between closed and open states. Could V434 have a role in maintaining Kv3.1 in the open-like state? You might think leucine and valine are not that different as amino acids go, what’s an extra methylene group among friends? It’s the difference between typical neurodevelopment and a neurodevelopmental disorder.
Eliana lives with her parents and her new baby sister in Canada. Phenotypically, she does not present as a typical DEE. However, she has significant hypotonia, cortical visual impairment, vertical nystagmus, and global delays. You could say genetic lightning struck again because Eliana’s mom Stephanie happens to be a genetic counselor by profession. Stephanie and her husband Chris sprung into action after Eliana’s diagnosis and founded the KCNC1 Foundation to drive research forward. Today, the Foundation is aware of 36 patients affected by a total of 14 variants: ~25% of patients share the same variant, A421V; 12.5% have MEAK caused by the R320H variant, which is thought to be an under-representation; a handful of patients have V432M; the rest of the variants affect 1-3 patients each.
Stephanie engaged Perlara for “cure guidance” a year and a half ago. Our core recommendation was to focus on drug repurposing as the fastest, cheapest and safest route to a clinically actionable intervention. Dr Whitney Dolan has served as what we call a generalist Cure Guide, providing comprehensive project management and continuity over time. Whitney is paired up with specialist Cure Guide Dr Van Duesterberg who got a PhD in biophysics studying ion channel function. We’ve found that this Cure Guide generalist-specialist duo model works well operationally across dozens of projects regardless of disease gene or therapeutic modality.
When we started working together 18 months ago, we weren’t certain whether Eliana’s V434L variant is loss-of-function or gain-of-function. Normally that’s a showstopper — but not in The Great British Baking Show sense. That thorny issue got sorted out earlier in the year by an academic group at the same KCNC1 Foundation elected to move forward with an unbiased V434L-focused drug repurposing screen in collaboration with Metrion Biosciences, a CRO that specializes in high-throughput ion channel screens.
7 months ago when we posted the last project update, the results of the Clatot et al study — the first to describe the V434L variant as having a gain-of-function effect on channel activity — had just been by reproduced by the experienced team of electrophysiologists at Metrion. The structural insights gleaned from the cryo-EM model were right: the mutant V434L Kv3.1 channel appears to open too easily and then have a hard time closing once open. Paradoxically, even though the Kv3.1 channel is more active, the neurons that express it become less active.
However in science, no result is sacred until it’s convincingly replicated and properly controlled. It was critical for Metrion to independently reproduce the published academic findings, which were performed on HEK293 cells that were transiently transfected with a plasmid expressing wildtype KCNC1 or one of three pathogenic variants: M430I, V432M, and Eliana’s V434L.
We realized early in the project planning stages that doing a drug repurposing screen in neurons differentiated from Eliana’s iPSCs would drive up costs substantially, as in tripling the budget, not to mention doubling (at least) the timeline. Clatot et al validated the minimum viable channelopathy model: a generic cell line expressing the mutant V434L Kv3.1 channel. One of the hoops that Metrion needed to jump through in the assay optimization stage was assessing if transient transfection would be sufficient or whether they would need to stably transfect cells with V434L KCNC1. They also needed to confirm which exact channel activity assay to deploy in a high-throughput screen. All doable based on a time-tested ion channel screening playbook.
The major caveat of the minimum viable channelopathy model is that a strictly electrophysiological readout will not capture intracellular trafficking defects or protein stability defects that result from the V434L gain-of-function variant. That said, it also doesn’t take much sleuthing on PubMed to find multiple examples of drugs that hit potassium channels like Kv3.1 as an off-target. Polypharmacology is no stranger to ion channels. Which brings us back full circle: centering the research process on Eliana’s V434L variant is the only way to make a data-driven decision since we can’t assume even neighboring KCNC1 variants have the same effects on Kv3.1 channel function.
In this section, we get in the weeds of high-throughput screening for the benefit of families and foundations looking to apply the minimum viable disease model playbook to their channelopathy. Metrion started out with transient transfection of CHO cells with plasmids expressing wildtype KCNC1 or Eliana’s gain-of-function variant V434L. As shown in the figure below, the transient transfections of CHO cells using plasmid encoding human KCNC1-WT and human KCNC1-V434L worked equally well, allowing progression to the biophysical characterization of both KCNC1-WT and KCNC1-V434L currents. Moreover, as an important negative control, non-transfected cells were shown not to exhibit electrophysiological activity confirming that the currents recorded from transfected cells were generated by human KCNC1-WT and KCNC1-V434L channels. So far, so good.
The FLIPR assay uses a fluorescent dye to measure ion flow through the Kv3.1 channel, thereby converting an electrophysiological readout into a fluorescence readout. The dye is sensitive to changes in intracellular thallium ions (Tl+), which are used as a surrogate for potassium ions (K+). The rate and quantity of Tl+ ions flowing into the cell can be regulated by opening and closing the Kv3.1 channels in the plasma membrane. An increase in fluorescence measured on the FLIPR directly correlates with an increase in Tl+ flux into the cell.
The V434L gain-of-function variant is activated at lower concentrations of K+. In a series of benchmarking experiments, Metrion characterized WT versus V434L Kv3.1 channels in response to two known Kv3.1 channel modulators: AUT1 and 4-aminopyridine (4-AP).
The application of 4-AP 3 mM almost completely inhibited the KCNC1 WT mediated currents, while the same 4-AP concentration only blocked the KCNC1 V434L current by 40%. The application of Aut1 increased the current amplitude when tested on the KCNC1 WT channel in a dose-dependent manner, while the application of Aut1 at 30 µM strongly blocked the current mediated by the V434L Kv3.1 channel. Altogether, these results indicate that the V434L Kv3.1 channel shows a different pharmacological profile when compared to the WT channel.
Assay performance of the transiently transfected polycloncal cells (meaning the cells were not derived from a single clone) was not ideal. We half-expected this outcome. So Metrion isolated monoclonal lines expressing either WT or V434L channels. As shown below, clone 2G6 showed the most reproducible behavior across 120 replicate wells.
Further QC and assay performance was done by Metrion prior to the primary screen of the Broad Repurposing Hub library. The spiked plate assessment is where a dilution series in triplicate is randomly distributed across the wells of a plate and then a dose-response curve is computationally reconstructed. Two known Kv3.1 channel inhibitors were assessed: AUT1 and the antidepressant fluoxetine (aka Prozac).
Well-fit dose response curves were successfully generated from the randomly distributed data points. AUT1 and fluoxetine are virtually indistiguishable and equipotent Kv3.1 channel inhibitors.
The IC50 of fluoxetine on V434L Kv3.1 channels is 18.5µM when the two replicates are averaged, modestly exceeding the plasma concentration achieved in clinical dosing in the on-label antidepressant context. That’s not what any pharmacologist would call potent inhibition. So the antidepressant pharmacology/mechanism of action of fluoxetine will certainly be in play. Perhaps neurogenesis is an on-target side effect?
Intriguingly, a recent paper described a nearby valine residue V425 in KCNC1 that is mutated to methionine (V425M) and results in a modest gain-of-function and electrophysiological profile somewhat similar to V434L, with the key and perhaps critical difference being that V425M Kv3.1 channels behave more normally when co-expressed with wildtype Kv3.1 channels.
Remarkably, a child with V425M has been taking fluoxetine as part of a 3+ year single-patient observational study in Italy. Seizures have been completely controlled and the child has experienced neurodevelopmental gains and psychomotor improvements. Will fluoxetine emerge as a top inhibitor of V434L? What other compounds will be revealed by an unbiased screen of the Broad Repurposing Hub library?
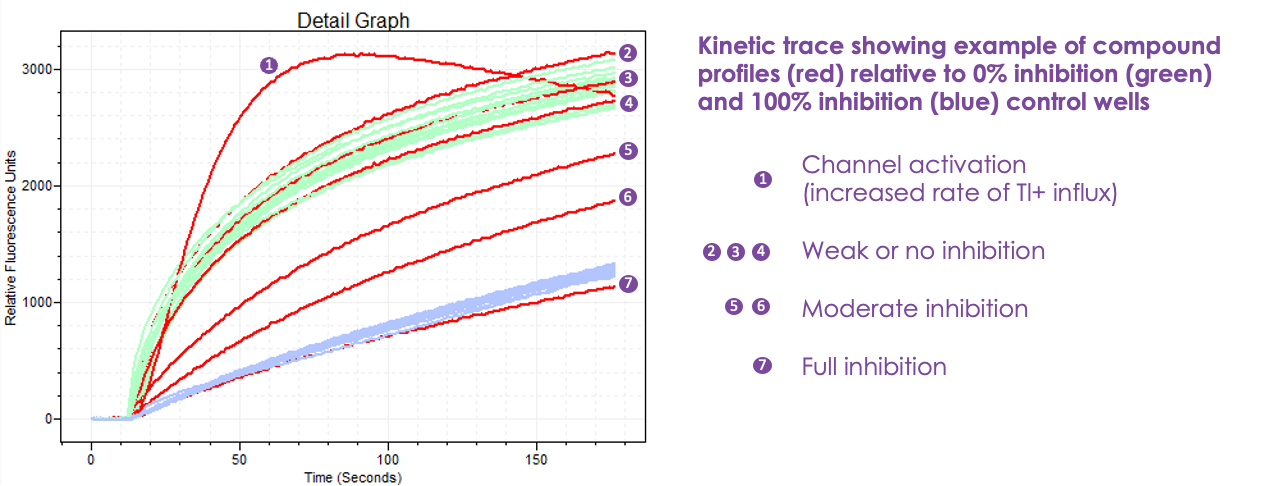
While much of Europe was sunning itself on a Mediterranean beach in August, Metrion received compound plates from the Broad Institute, with plans to begin screening the last week of August. Compounds were screened at 10 µM in duplicate. Below is an example the FLIPR assay output in relative fluorescence units. The green traces indicate 0% Kv3.1 channel inhibition (aka the negative control) while the blue traces indicate 100% Kv3.1 channel inhibition (aka the positive control).
The goal of this precision drug repurposing campaign is to identify V434L Kv3.1 channel inhibitors that behave like the red trace 7, i.e., full inhibition.
For high-throughput screening aficionados, the signal-to-background (S:B) ratios and the Z-prime values (RZ’) of each replicate pair of plates comprising the entire Broad Repurposing Hub library are plotted below. Well-performing screens have a S:B ratio greater than 3, and RZ’ greater than 0.5. Metrion easily cleared that hurdle.
The Broad Repurposing Hub library fills 23 384-well plates. One way to visualize the concordance between the two replicates is by using plate heatmaps where the intensity of channel inhibition or activation by each test compound in a well corresponds to the color intensity of red and blue, respectively. Test compounds that have no effect on V434L Kv3.1 channel function are rendered in white.
Here’s the distribution of test compounds plotted as a function of percent inhibition. Several dozen test compound inhibit V434L Kv3.1 channel function as well or more as the positive control. Reassuringly, hit compounds are randomly distributed across the library and not clustered in a handful of plates.
Now let’s merge the test compounds from the two replicates into a single plot while subtracting out the control wells. When the threshold for inhibition is set at 90%, there are 34 test compounds that hit the mark in both replicates. That translates to a hit rate of 0.5%, the sweet spot for a robust unbiased phenotypic screen.
What happened with fluoxetine? Turns out fluoxetine and its primary active metabolite norfluoxetine are both present in the Broad Repurposing Hub library. Based on the results from the assay optimization stage, we expected at least 50% inhibition by fluoxetine at the 10 µM dose. However, what we observed was an underperformance for whatever reason. The average inhibition by fluoxetine was 7% (4.7% in replicate 1 and 9% in replicate 2). The average inhibition by norfluoxetine was -6.4% (technically slight channel activation) in replicate 1 and 3.4% in replicate 2, meaning no difference from control.
While there’s still justification for trying fluoxetine in a V434L single-patient study based on the successful V425M single-patient study, the V434L-focused drug repurposing screen produced one more surprise. There’s a safe, over-the-counter nutraceutical (marked by the yellow arrow on the plot below) that caused 112% inhibition of the mutant V434L Kv3.1. channel in both replicates. Now that the primary screen is done, the next and final stage of the screening campaign is hit validation and potency determination studies. We are also considering a test of selectivity, i.e., do the top hits inhibit WT channels as potently as V434L channels?
We’re excited to work with KCNC1 Foundation and a clinician to boot up a single-patient study for Eliana based on the above results. All eyes had been on fluoxetine until the Broad Repurposing Hub library screen was finished last month. We can only test one drug at a time in pioneer patients. And of course it’s possible that combo therapy will be superior to a monotherapy.
Onward for Eliana and KCNC1 kiddos like her!



















Hi Authors, wonderful article, I work in marketing at Metrion Biosciences, the CRO partner on this programme, please can I share the details of the project within a case study? Warm regards, Sophie