MEPAN yeast drug repurposing updates
Last time we reported that antifungal drugs are top hits from drug repurposing screens of yeast models of a rare mitochondrial disease. Hit validation in flies and fibroblasts is nearing completion.
In the last MEPAN yeast drug repurposing project update, we shared the unexpected discovery that a specific class of antifungal drugs are the top hits that rescue growth of MEPAN yeast avatars, or mutant strains harboring a particular pathogenic MECR variant.
Five MECR variants were modeled as yeast avatars and then the Pharmakon collection was screened for compounds that rescue growth in conditions that require functional mitochondria (note that the human MECR gene counterpart in yeast is ETR1):
Y285C (missense mutation)
R258W (missense mutation)
G232E (missense mutation)
Y285* (nonsense mutation)
Asn83Hisfs*4 (frameshift mutation)
The missense mutations destabilize the enzyme, but in theory some amount of mutant protein is still produced by the cell and is amenable to enzyme activation via direct protein stabilizing mechanisms such as pharmacological chaperoning or via indirect mechanisms such as increasing enzyme cofactor abundance or blocking enzyme degradation, among others. Alternatively, a hit compound can rescue growth by activating a bypass mechanism that operates in the absence of MECR enzyme.
In hit validation studies, we included a MECR/ETR1 whole-gene knockout mutant so we could compare the three missense hypomorphic mutants relative to the nonsense and early frameshift mutants, which are both predicted to be yield a non-functional protein if any protein at all.
Three hits stood out in pair-wise Z-score comparisons of the MEPAN yeast avatars: anidulafungin, micafungin and caspofungin.

These three drugs comprise the echinocandin class of broad-spectrum antifungals, which are routinely prescribed for Candida and other fungal infections. Notably they are on the World Health Organization’s List of Essential Medicines, generally considered safe, and approved for use in adults and in children as young as one month. They are administered by intravenous (IV) infusion.
Do they get into the brain? Only a few studies have examined this question in humans. The short answer is yes, but much less exposure is achieved in the brain compared to other organs. As we’ll show, the therapeutic dose in MEPAN yeast avatars appears to 10-100 fold lower than the clinical antifungal dose, consistent with the idea that echinocandins have a second and heretofore unrecognized pharmacology revealed by unbiased yeast avatar growth rescue screens.
Echinocandins are natural product cyclic hexapeptides with a long-chain fatty acid tail produced by fungi and first discovered in the 1970s. They have been described as the “pencillin of antifungals” because they inhibit the glycosyltransferase enzyme that assembles an essential brick used in the production the yeast’s proteoglycan cell wall. Semi-synthetic echinocandins have been in medical use for over two decades, starting with caspofungin (FDA approved since 2001) followed by micafungin (FDA approved since 2005) and anidulafungin (FDA approved since 2006).
Rezafungin is an investigational fourth-generation echinocandin derived from anidulafungin that has an improved safety profile and a once-weekly dosing schedule. It’s in Phase 3 clinical development and a NDA has been filed by the commercial sponsor with regulatory approval likely next year.
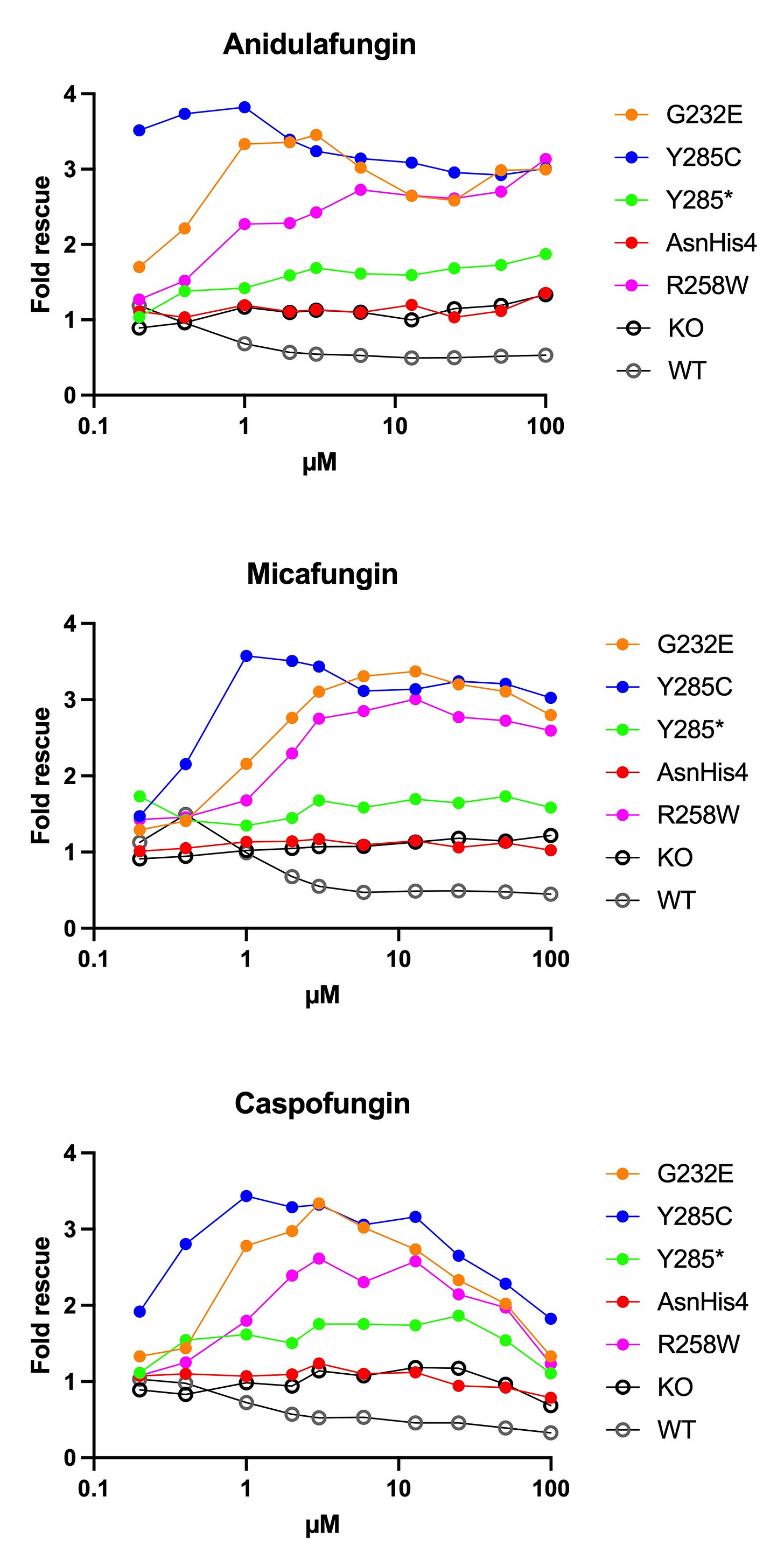
The first round of dose response data that we reported in August were not as clean as we would have liked so the experiments were repeated. Below are growth rescue results from MEPAN yeast avatars expressing one of five different pathogenic MECR variants and treated with an echinocandin drug in media containing ethanol as the sole carbon source.
The ETR1 knockout yeast mutant strain (“KO”) was included to test whether growth rescue requires the presence of the ETR1 protein. The answer is, yes. In the absence of ETR1 protein, none of the echinocandins rescue growth in ethanol. Knocking out ETR1 also confers resistance to the echinocandins. The wildtype (“WT”) strain was included to determine the minimum antifungal dose, which appears to be 1µM. Growth rescue at the two doses below 1µM is consistent with a second pharmacology.
Several observations are noteworthy. There is no rescue of the KO and AsnHis4 frameshift mutant, which appear indistinguishable. There appears to be very modest rescue of the Y285* nonsense mutant, but nowhere near the degree of rescue afforded the three missense mutants. Across all three echinocandins, the order of rescue is: Y285C > G232E > R258W. Across all three missense mutants, the order of potency is: anidulafungin > micafungin > caspofungin.
Caspofungin’s antifungal effect cancels out its growth rescue effect at 10µM and higher doses. Notably, anidulafungin has rescue effects on missense mutant growth at nanomolar doses where it has no effect on WT or KO growth, and below the antifungal minimal inhibitory concentration. Altogether, anidulafungin appears to be the most potent and broadly acting of the three echinocandins.
Collectively, the yeast data strongly suggest that echinocandins cause activation of MECR. The exact mechanism of action is still unknown. It’s possible that we’re underestimating echinocandin polypharmacology, especially in more complex species. Experiments assessing rescue by echinocandins of wall climbing ability of a MEPAN fly and, in parallel, rescue by echinocandins of protein lipolyation and bioenergetics defects in MEPAN patient fibroblasts are ongoing. Data readouts are expected soon..