Low-dose statin as a potential treatment option for SRD5A3-CDG
Sappani Foundation created a worm avatar for drug repurposing in 2019. After many twists and turns, HMG-CoA reductase emerged as a target that may restore the balance between polyprenol and dolichol.
In collaboration with
Disclaimer
The results of the SRD5A3-CDG drug repurposing project that we are sharing below in the spirit of open science are novel preclinical research findings and therefore they do not constitute the practice of medicine. Please consult your physician or clinical care team if you’re considering off-label use of any approved drug. The same caution applies to nutraceuticals, supplements and “generally recognized as safe” compounds.
Longtime readers of Cure Odysseys (and its predecessor The Ark) know that CDGs — Congenital Disorders of Glycosylation — are one of our core areas of focus, starting almost a decade ago with NGLY1 deficiency. Working alongside families of the first kiddos diagnosed with the disease, we launched an open science collaboration we called the NGLY1 PerlQuest, the precursor to what we now call a cure odyssey.
In late 2016, NGLY1 pioneer Dr Matt Might introduced us to PMM2 pioneer Holly Carmichael, which led to the launch of the PMM2-CDG PerlQuest a few months later. We’ve been working with pioneer CDG families ever since.
In early 2019, we learned about SRD5A3-CDG from Vijay and Abi Sappani and their daughter Ela. Just as Perlara 1.0 was beginning a metamorphosis into Perlara 2.0: from a centralized biotech company whose platform technology was model organisms aka patient avatars into a fully distributed and modality agnostic biotech company where the patients are the platform. In these specific cases, parents working to cure their kids are the platform.
Ela is one of about 50 patients in the world with a confirmed genetic diagnosis of SRD5A3-CDG, one of the 170+ subtypes of CDG. SRD5A3-CDG is predominantly caused by homozygous loss-of-function mutations in the SRD5A3 gene and was first described as a novel CDG in Cantagrel et al., 2010.
Ela inherited two copies of the W19X nonsense variant, which means she’s as close to a whole-gene SRD5A3 knockout as you can get. And she’s not alone because W19X is a recurring variant. SRD5A3 encodes an enzyme called steroid 5ɑ-reductase 3 aka polyprenol reductase, which converts polyprenol to dolichol.

Let’s zoom in on the dolichol synthesis portion. SRD5A3 is fueled by the mighty mevalonate pathway. Imagine a massive melting spring snowpack that feeds many downstream rivers and tributaries. If the absence of SRD5A3 does not primarily affect dolichol levels because there’s some amount of built-in compensation by a cousin enzyme like SRD5A2, then disease pathology may be a consequence of an unhealthy accumulation of unreduced polyprenols.
In other words, as Grundahl et al., 2012 originally theorized, perhaps the primary disease driver is the ratio of polyprenol to dolichol?
If too much polyprenol runs amok in the cell, it will compete with dolichol for precious nucleotide sugars like GDP mannose and other N-glycosylation building blocks. That should lead to a global reduction in N-linked protein glycosylation across the glycoproteome. At the same time, too much polyprenol might also create acute dolichol shortages in specific cell types and/or under certain physiological conditions.
Therefore, it is not unreasonable to postulate that reducing flux through the mevalonate pathway would provide relief when SRD5A3 activity is diminished or absent. The key is to reduce flux through the pathway, not shut it off entirely. Otherwise the cell will impoverish ubiquinone, cholesterol and steroid production. The key question is: what is a safe “rebalanced” flow rate of the mevalonate pathway in SRD5A3 deficiency?
Inspired by the PMM2-CDG and NGLY1 PerlQuests, Sappani Foundation and CureSRD5A3 embarked on a multi-species drug repurposing journey for SRD5A3-CDG. In mid-2019, a Canadian biotech startup called Modelis was commissioned to generate and characterize Ela worm avatars, and then deploy them in drug repurposing screens.
Modelis generated an Ela worm avatar and subjected it to a battery of tests. Think of it as the worm version of a natural history study, except greatly sped up and miniaturized. We suspected that Ela’s W19X variant, which would result in a 19-amino acid severely truncated protein, would be equivalent to a whole-gene deletion aka knockout. So we included a SRD5A3 knockout avatar control side-by-side with the homozygous W19X/W19X Ela worm avatar.
Contrasting with NGLY1 deficiency and PMM2-CDG, we didn’t need to stress the SRD5A3 -CDG worm avatars to reveal a phenotype. The developmental delay observed in SRD5A3-CDG patients is mirrored in the worm avatars. Wildtype worms (N2) reach advanced larval stages (L3 and L4) but both the SRD5A3 knockout and Ela avatars fall behind and remain in mid-larval (L1 and L2) stages.
Consistent with having the worm equivalent of global developmental delay, SRD5A3 knockout and Ela avatars are smaller/shorter than wildtype worms at the late larval (L4) stage and as young adults (Days 1 and 5).
Next, we asked if SRD5A3 knockout and Ela avatars produce the same number of progeny when individual worms reach adulthood. Both avatars are much less fertile than their wildtype counterpart. The SRD5A3 knockout avatar and the Ela avatar are statistically indistinguishable from each other.
Next, we evaluated lifespan, which mercifully is not too long an experiment with animals whose days are numbered. As we found in the previous two vital assessments, both the SRD5A3 knockout and Ela avatars die about a week sooner than wildtype worms.
As a functional activity measure not directly related to growth and developmental timing, we monitored the motility of the SRD5A3 knockout and Ela avatars over the timescale of minutes. There is indeed a robust motility defect that Modelis exploited in a high-throughput primary drug screen to identify repurposing candidates that rescue the swimming deficit.
After multiple rounds of primary and secondary screens, we settled on four clusters of hits:
antihistamines
NSAIDs
HMG-CoA reductase (HMGR) inhibitors
antipsychotics
We were intrigued by the HMGR inhibitor cluster anchored by atorvastatin aka Lipitor. Dietary cholesterol represses HMGR via negative feedback inhibition. Several plant-based polyphenols — irigenin, syringetin-3-glycoside, isotectorigenin, and hypocrellin A — also rescued the swimming deficit of the Ela worm avatar. What do they all have in common?
As show in the figure below, they all contain complete or incomplete mevalonate pharmacophores. Mevalonate spontaneously and reversibly isomerizes to mevalonolactone. Remarkably, both acylic and cyclic mevalonate and mevalonate-like pharmacophores are observed among the SRD5A3 worm hits.
To prove we aren’t hallucinating the mevalonate and mevalonate-like pharmacophores, the levels of metabolites in the mevalonate pathway were directly measured by LC/MS (liquid chromatography/mass spectrometry) in the SRD5A3 worm avatars and normalized to the levels measured in the wildtype control.

The following mevalonate metabolites changed in the Ela worm avatar:
↓ = HMG-CoA, mevalonate, isopentyl-PP, dimethylallyl-PP, total dolichols
↑ = mevalonate-PP, farnesyl-PP, GGPP, ubiquinone-11
The following cholesterol metabolites changed in the Ela worm avatar:
↑ = oxidosqualene, lanosterol, dehydrolathosterol, dehydrodesmosterol
Those changes are consistent with a rerouting of mevalonate flux away from the dolichol/N-glycosylation shunt and towards the other major isoprenoid tributaries, namely cholesterol and ubiquinone production. We’ll return to the SRD5A3 worm avatars after an interlude with patient fibroblasts.
Shifting gears from worms to human cells, we turned to the laboratories of Dr Eva Morava-Kozicz and Dr Akhilesh Pandey at the Mayo Clinic to perform validation studies on five different SRD5A3-CDG patient-derived fibroblast lines. Almost every SRD5A3-CDG patient has nonsense mutations or indels.
As a sanity check, proteomics experiments by the Pandey lab demonstrated that the expression of SRD5A3 protein (red dot in the volcano plot below) is indeed statistically significantly reduced in SRD5A3-CDG patient fibroblasts relative to age-matched control fibroblasts.
Next, we found evidence that SRD5A2 protein levels are increased in SRD5A3-CDG patient fibroblasts compared to control fibroblasts. This result obviously needs to be confirmed in other human cell types, e.g., neurons, but it suggests that SRD5A2 has the capacity to serve as an imperfect backup polyprenol reductase.
Next, Pandey lab performed glycoproteomics analysis and found that the abundance of all types of glycopeptides is decreased in SRD5A3-CDG patient fibroblasts. The lower abundance aka hypoglycosylation is indicated by the leftward spray of points in the volcano plots below.
Then Pandey lab performed a final set of lipidomics experiments on the SRD5A3-CDG patient fibroblast lines and the age-matched control fibroblasts lines to determine the total dolical/polyprenol ratio. As expected, the ratio is lower in cases versus controls because polyprenol levels (the denominator) is increasing while the dolichol levels (the numerator) stays the same.
The worm and fibroblast data agree that the mevalonate pathway is out of balance in SRD5A3-CDG. After many twists and turns and several years on this journey, we just received hit validation data from the worm progeny count assay, but we’re still a few weeks away from receiving the LC/MS analysis of the mevalonate pathway in response to treatment of worms with the SRD5A3 worm hits.
As of this moment, we’re cautiously optimistic about low-dose atorvastatin, which has a rescue effect at 0.1µM but has a sensitizing effect at the highest doses, 50µM and 100µM.
Intriguingly, piroxicam, an NSAID, also rescued progeny counts at 1µM and 10µM doses. None of the other eight SRD5A3 worm hits from the primary screen validated in this more stringent worm progeny count assay.
Based on the totality of the data, we summarize the case for partial inhibition of HMGR as a potential therapeutic strategy for SRD5A3-CDG. Low-dose statin therapy is the most pharmacologically targeted and tractable approach, followed by dietary cholesterol. The plant-based polyphenols (isoflavonoids) have additional pharmacological mechanisms beyond HMGR inhibition that make assessing and dosing them challenging, and so they would require further head-to-head and combination testing with a low-dose statin.
Statins have been used in children with other rare genetic diseases such as hypercholesterolemia and Smith-Lemli-Opitz syndrome (SLOS). Nevertheless, we absolutely stress the need for a standardized and streamlined clinical protocol that would allow for objective monitoring of safety endpoints and exploratory efficacy endpoints of low-dose statin monotherapy in single-patient studies of patients living with SRD5A3-CDG.
























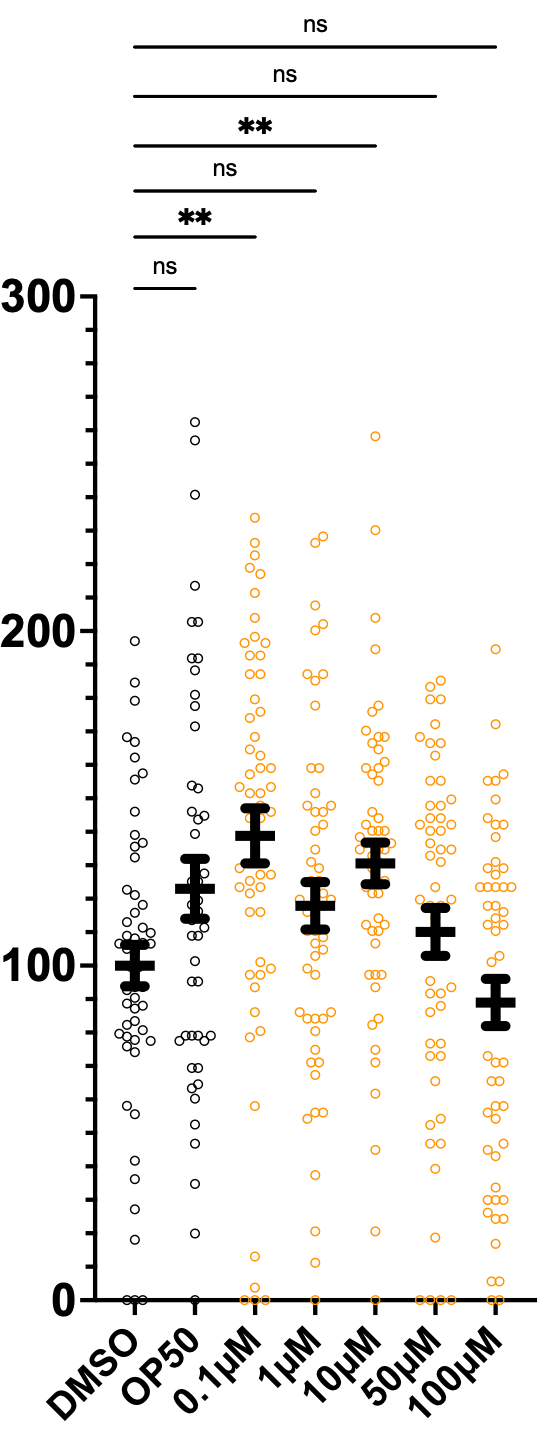

you may also want to supply bile acids in addition to cholesterol when try statin. Good luck
One of my concerns with them is that they might increase Glycanage, b/c N-linked glycosylation is important for the initial steps in forming more complex glycans
https://www.rapamycin.news/t/statins-often-used-to-reduce-rapamycin-increased-ldl-inhibit-coenzyme-q10-and-ggp-mk4-and-dolichol-and-n-linked-glycosylation-other-negative-effects/13775/9