Two nutraceuticals are the top drug repurposing hits for ALG11-CDG
An initial pioneer ALG11-CDG family blazed a trail with a pilot 2,200-compound screen, paving the way for a second ALG11-CDG family to grab the baton and fund a comprehensive 8,400-compound screen.
In collaboration with
Steadfast readers of Cure Odysseys will recognize ALG11-CDG as one of the 12 congenital disorders of glycosylation (CDG) drug repurposing programs in Perlara’s direct-to-patient, family-driven pipeline which we affectionately dub the Anti-Portfolio of Pharma.
NGLY1 deficiency is Perlara’s first CDG program. Aripiprazole (Abilify) was the last hit standing after a three-species — worm, fly, human cell — drug repurposing campaign. Abilify has so far been trialed in three NGLY1 kiddos, including one patient with a durable positive response to treatment. Epalrestat for the treatment of PMM2-CDG is the most advanced CDG program to emerge from Perlara’s pipeline. What began in 2017 as a PerlQuest collaboration is now a standalone drug development company called Maggie’s Pearl, established in August 2020. Maggie’s Pearl is the commercial sponsor of a fully enrolled pivotal Phase 3 trial currently in the open label stage.
Eight of Perlara’s 12 CDG programs moved from screening hits in a model organism to testing in humans. The 1-to-N medicine model we’ve pioneered is on the precipice of scaling. That scale is made possible by an unexpected observation that we didn’t predict at the outset (though in retrospect it’s kind of obvious). In 6 out of 12 CDG programs, at least one clinically actionable nutraceutical was among the top 10 hits, if not the top hit.
By “clinically actionable” we mean available without a prescription — over-the-counter — which could be a generic drug like the NSAID ibuprofen for MAN1B1-CDG or a nutritional supplement like alpha-lipoic acid for PGAP3-CDG. The catchall term “nutraceuticals” runs the gamut from bioavailable forms of naturally occurring metabolites (or metabolic precursors) to bioavailable forms of natural products like plant-based polyphenols.
In the case of ALG11-CDG, we believe we’ve identified what fits the definition of a novel therapeutic: a broad term which in this case refers to a specific and nonobvious combination of two nutraceuticals that could not be predicted by any AI model — at least for now. In fact, it’s the collective “Organic Intelligence” of billions upon billions of yeast cells that narrowed down a library of 10,000 compounds to just two compounds that demonstrate a reproducible rescue effect.
A year ago we summarized our disease modeling experience working with an initial ALG11-CDG pioneer family. You can get caught up to speed here. We ended the last installment with a cliffhanger: we were about to screen the Pharmakon library. Before we get to the results, a quick refresher on ALG11.
ALG11 is located in a part of the N-glycosylation supply chain that we haven’t yet explored as much as the GPI anchor biosynthesis and dolichol biosynthesis branches. In fact it’s the only ALG program we’ve worked on to date!

ALG11 forms a mannosyltransferase complex with partner proteins ALG1 and ALG2. The nascent glycan chain receives its first mannose additions at this step in the supply chain. We’ve worked on mannose metabolism itself in the form of PMM2-CDG and GMPPA-CDG, which fall under the umbrella of nucleoside sugar synthesis. Our intuition is that the metabolic rebalancing mechanisms at work in other CDGs will also manifest in ALG11-CDG. The question is, which specific ones?
We might expect based on our experience with PGAP3-CDG that a compensatory enzyme can moonlight in the stead of the hobbled enzyme. In this case another phospholipase, maybe one that doesn’t normally prefer PGAP3’s substrate but can be coaxed to substitute for PGAP3 under the right conditions.
The solution may be in the substrate, not the enzyme. If mannosyltransferase enzymatic rates are decreased, does mannose metabolism need to be decreased in lockstep in an attempt by the cell to maintain homeostasis, like we saw in the case of HMG-CoA inhibitors (statins) rescuing disease models of SRD5A3-CDG, where polyprenols accumulate to toxic levels?
One can even imagine a third class of rescue mechanism that is a counterintuitive inverted mirror of a substrate reduction model. A substrate surge approach, if you will, whereby flux of an upstream rate-limiting metabolite is increased to compensate for the reduction of a downstream rate-limiting metabolite. Perhaps that surge is an orthogonal attempt by the cell to restore homeostasis by juicing a moonlighting enzyme or bypass synthesis pathway.
The reality is likely complicated. All three rebalancing mechanisms may contribute to rescue in ALG11-CDG. The only way to find out is to run some screens.
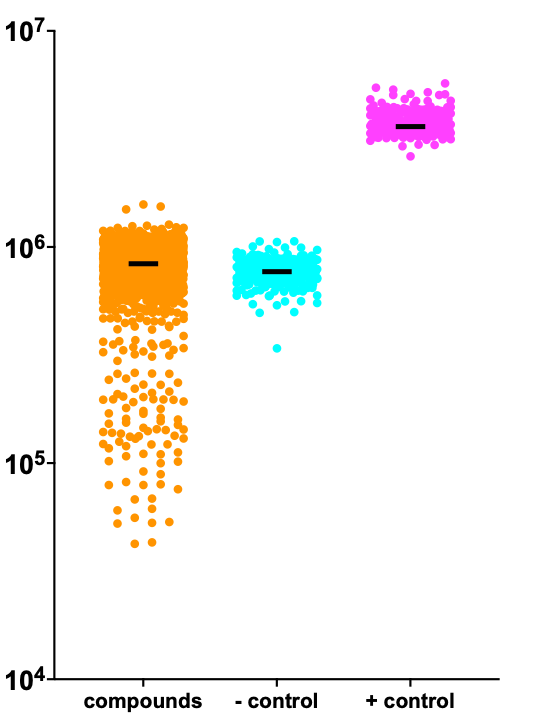
Recall that the growth of the ALG11 knockout yeast avatar could be finicky. In the Pharmakon screen, the ALG11 knockout yeast avatar grew better than expected, so the delta between the negative control and the positive control was smaller than the deltas in other Pharmakon screens. Despite the higher baseline growth of the ALG11 knockout yeast avatar, there was room for a few rescuers to separate from the pack. A notably large contingent of sensitizers was observed.
The ratio of rescuers to sensitizers is markedly skewed toward sensitizers. There are only a handful of compounds that make the ALG11 knockout yeast avatar grow better, but many more compounds that make the ALG11 knockout yeast avatar fare worse. In genetics speak, complete loss of ALG11 is difficult to suppress but relatively easy to enhance. When interpreting these findings, keep in mind that patients living with ALG11-CDG have some residual ALG11 enzymatic activity versus none in the ALG11 knockout yeast avatar.
We had similar issues wrangling the PIGA-CDG yeast avatar, whose growth defect was extraordinarily sensitive to temperature. Off by less than one degree centigrade, the growth defect is lost. A comparison to other Pharmakon screens reveals that ALG11-CDG and PIGA-CDG have similarly shaped distributions.
Both show a pronounced skew toward sensitizers relative to the number of rescuers, PIGA-CDG much more so than ALG11-CDG. By contrast, the ratio of rescuers to sensitizers is balanced in the cases of PIGN-CDG and PGAP3-CDG. By contrast, the mitochondrial disease gene SURF1 has an excess of rescuers relative to the number of sensitizers.
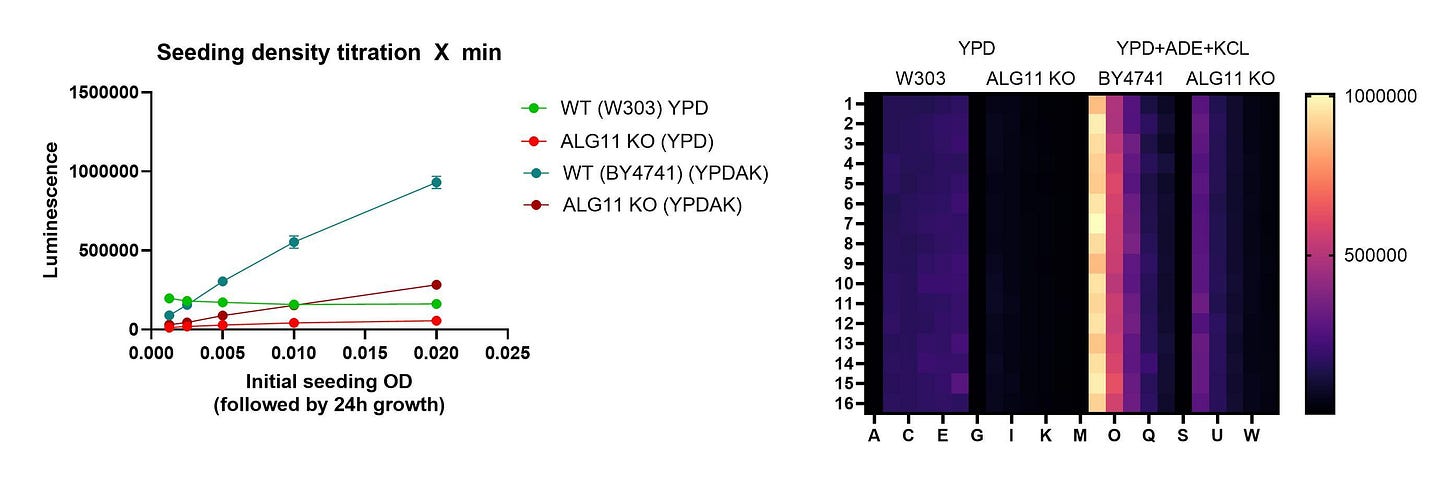
Unlike PMM2 and the PIG genes, the ALG11 gene is not essential for yeast growth at standard growth conditions. Lethality is only revealed under high-temperature stress. The ALG11 knockout yeast avatar — the upper-left plate — cannot grow at 37˚C.
We encountered road bumps working with ALG11 yeast avatars in the past. So in seeding density experiments we knocked out the ALG11 gene in two different yeast strains (W303 and BY4741). We also tested growth in two different media. We had to get the conditions just right, otherwise the bake would be off.
Z-score distributions of the 8,400-compound TargetMol library screen are shown below. Reassuringly, a nearly identical distribution of hits was observed in both the Pharmakon and TargetMol screens, which were completed at different times and locations. We also observed the familiar skewed ratio of sensitizers to rescuers.
There was a runaway jackpot hit that grew better than wildtype yeast, the positive control. We were immediately suspicious, as we’ve seen exaggerated screen winners like this before. However, three moderate rescuers, and about a dozen modest rescuers, caught our eye.
We ordered 12 hits for hit validation and potency determination studies. The runaway was indeed too good to be true. It showed absolutely no effect in a dose response assay.
The clear winner with a robust rescue effect is a potential novel therapeutic (Compound 2), which happens to be the overall second-ranking hit in the TargetMol screen once the false positives are removed from consideration. The only other hit that validated has a much more modest rescue effect (Compound 1), which is a plant-based nutraceutical available as an over-the-counter direct-to-consumer product.
Next steps are to pursue mechanistic studies in ALG11-CDG patient fibroblasts for both top hits, and to start a n-of-1 observational study with the plant-based OTC nutraceutical.