All in the FAMily
An unbiased fly-powered drug repurposing screen for FAM177A1 deficiency turned up the generic ALS drug riluzole as the top hit and inspired a family-initiated, family-led pioneer N-of-1 study.
In collaboration with
Disclaimer
The results of the FAM177A1 drug repurposing project that we are sharing in the spirit of open science below are novel preclinical research findings and therefore they do not constitute the practice of medicine. Please consult a physician or clinical care team if considering off-label use of any approved drug or compassionate use of any experimental drug. The same caution applies to nutraceuticals, supplements and “generally recognized as safe” compounds.
One day a mighty AGI oracle will effortlessly divine drug repurposing recommendations for the long tail of 10,000+ rare diseases. We are already seeing glimmers of that promised land on the still faint horizon. So what are rare disease families with sick kids supposed to do today?
Especially in cases of rare diseases that are caused by a gene whose function has not yet been fully elucidated at the protein level. Zero-shot learning on data from related genes or symptoms will only take us so far when the long tail of biology is terra incognita and full of uncharted surprises. The same symptom can arise via multiple distinct mechanisms. No knowledge base, no knowledge graphs. No appropriate training data, no ready-made AI solution.
Take the example of the protein encoded by the FAM177A1 gene, where two loss-of-function mutations lead to a complex neurodevelopmental and progressive neurodegenerative disease. When we started working with Jill and Doug Hawkins, founders of the FAM177A1 Research Fund, and parents to two affected children Charlotte and Cooper, the FAM177A1 knowledge base was sparse — and that’s being generous.
A mere seven papers about FAM177A1 had been published in January 2023 when we set out on the drug repurposing leg of the multi-modality FAM177A1 cure odyssey. Only one of those seven papers involved a gene-specific investigation of FAM177A1 function. The rest were casting a wide genome-wide association study net and hauled in FAM177A1 along with other candidate disease-causing genes. Not to mention that the FAM177A1 protein has unstructured domains whose functions are anyone’s guess.
Over the past 18 months, three new attention-worthy FAM177A1-focused papers have emerged, filling key gaps in the FAM177A1 knowledge base. The first paper is a formal description of FAM177A1 deficiency as a rare genetic disease and includes disease modeling in patient fibroblasts and a zebrafish model (Kohler et al., 2024).
The second paper is a cell-biology-oriented study that independently confirmed FAM177A1 protein localization to the Golgi and also showed that FAM177A1 appears to be involved in lipid trafficking based on its association with a known Golgi lipid trafficker, VPS13B (Ugur et al., 2024).
Intriguingly, a third paper centered on a population-wide association study found that higher expression of FAM177A1 protein is associated with protection from a progressive autoimmune disease called primary biliary cholangitis, where lipid trafficking specifically runs amok in the liver (Yang et al., 2023).
In order to keep filling in the fragmentary albeit slowly expanding FAM177A1 knowledge base, we applied some Organic Intelligence, or OI. At Perlara we love all model organisms equally. Choosing between them can be like picking a favorite child. The evolutionary history of the FAM177A1 gene made the selection process easy. Yeast and worms don’t have orthologs (versions) of the FAM177A1 gene, but flies do! So we connected Jill with Professor Clement Chow’s lab to discuss the possibility of running a drug repurposing screen in a fly model of FAM177A1 deficiency.
Two previous fly-powered drug repurposing screens, MAN1B1-CDG and GMPPA-CDG, led to the discovery of at least one clinically actionable hit. Ibuprofen in the case of MAN1B1-CDG, which was trialed in three parallel family-directed N-of-1 observational studies done in close consultation with physicians. In both of those instances, the fly version of the human disease gene had not be studied before. For our purposes, that doesn’t matter. All we need for an unbiased drug repurposing screen to work is a robust rescuable phenotype.
The project with Chow lab got rolling about a month after our introductory call. Initially, the Chow lab generated a series of RNAi knockdown models of FAM177A1 deficiency in flies. A robust “rough eye” phenotype was observed right off the bat for MAN1B1-CDG, so that’s where we looked first.
By April 2023, Chow lab had tried three different FAM177A1 RNAi lines, each line with a different eye driver, and incubated flies at two temperatures. Alas, they found no effect on the fly eye. They even tried incubation at 30˚C or “heat shock” to stress the flies and force a rough eye phenotype, but no dice. So they pivoted to whole-animal survival as a readout.
Case in point: in the GMPPA-CDG screen, we successfully rescued a strong lethality phenotype. Chow lab tested if lethality could be induced by knocking down FAM177A1 ubiquitously, i.e., in all cells of the fly, or only in restricted tissues or cell types such as neuron, glia, muscle or wing.
By June 2023, five months into an unexpectedly protracted model qualification process, we were getting nervous that we might have hit a dead-end. Then one day we were relieved to receive this email from Prof Chow:
“This was the most difficult pre-screen analyses we’ve had to date. But, the good news is, we can do a screen. The most robust, reproducible phenotype is a male-based lethality when we eliminate FAM117A1 expression in the muscles. 100% of all males die before adulthood – it’s very reproducible. There is lethality in the females as well, but it’s not 100% and varies a bit from trial to trial (not ideal). So we can run the screen based on whether we see any living males, which will make it robust.”
It was finally go time.
A representative live wild-type adult fly and two representative dead FAM177A1 knockdown flies are shown in the image below. The drug repurposing screening campaign took flight in the summer of 2023.
A screen of a ~1,400-compound tried-and-true drug repurposing library was completed and results were delivered in January 2024, a year after we made first contact. We screened that same library on MAN1B1 knockdown flies. We also screened that library on GMPPA knockdown flies, where rescue of lethality, i.e., whole-animal survival, was the chosen robust rescuable phenotype. However, instead of whole-body knockdown, which wasn’t as penetrant, muscle-specific knockdown of FAM117A1 in male flies did the trick for some reason.
A prodrug form of riluzole called troriluzole is being developed by Biohaven for several therapeutic areas including spinocerebellar ataxia (SCA) with a positive Phase III trial completed recently completed. SCA shares clinical symptoms with FAM177A1 deficiency. Troriluzole was designed to improve both bioavailability and safety, allowing a more consistent exposure to the parent compound riluzole.
Using a cutoff of 45% survival, we identified 33 hit compounds, or rescuers of lethality, which are summarized in the table below. Strikingly, half of the hits have pharmacology associated with action on acetylcholine signaling and metabolism. Other notably actionable compounds in the top 20 hits include allopurinol, loperamide and ranitidine, all three being safe and well-tolerated generic drugs. We always keep our eyes peeled for over-the-counter nutraceuticals and supplements. Pyridoxine brought up the rear with 46% rescue of lethality.
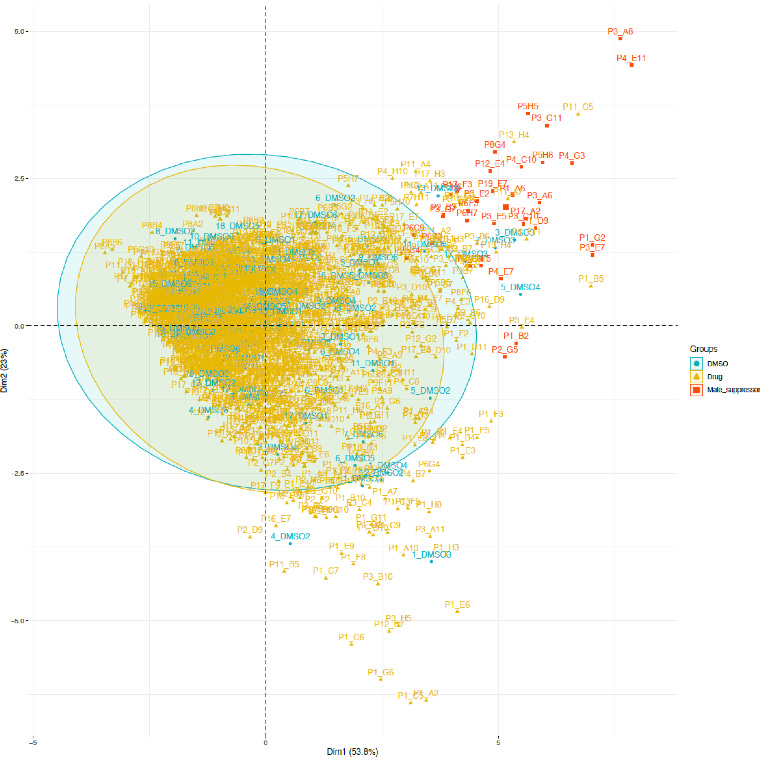
A PCA plot for FAM177A1 screen was done to understand how the hits bucket into mechanistic classes. The 33 hits with > 45% rescue of lethality in the primary screen are denoted in red. Almost all are on a vector in PCA space pointing concentrated in the upper right quadrant.
The upper right quadrant corresponds to male and female survival, the outcome we desire. Compounds that lie on undesirable vectors are eliminated from consideration. For example, compounds populating the upper left quadrant are doing the opposite of what we want: precocious lethality at the pupal stage or reduced fecundity.
From the 33 primary screen hits we down-selected to 11 compounds for dose-response hit validation experiments. This post-screening phase began in March 2024 and was wrapped up by May. Three doses were tested: 1 µM (low), 5 µM (medium) and 25 µM (high). Asterisks indicate statistically significant (p < 0.05) treatment effect.
Allopurinol and loperamide rescued at all three doses. Riluzole rescues at the 1 µM and 5 µM doses, but the rescue effect is lost at the highest dose, indicating dose-limiting toxicity at 25 µM. Pyridoxine is less potent with rescue only at the 5 µM dose, but dose-limiting toxicity at 25 µM. Ranitidine rescues at 1 µM and 25 µM, but strangely not at 5 µM. The other compounds either had either no effect (atracurium and clomipramine) or increased lethality at one or more doses (phentolamine, cyproheptadine, ethosuximide, linezolid).
In total, 5/11 primary screen hits validated, including the screen first-place winner riluzole. A 50% hit validation rate is consistent with our historical average.
Why did some rescuer compounds from the primary screen end up making the FAM177A1-knockdown flies worse in the hit validation experiments? Are they false positives, which pop up in any screen? There was only one statistically significant dose that caused increased lethality. Phentolamine and ethosuximide increased lethality at the highest dose (25 µM), which could be attributable to off-target toxicity.
We consider these academic questions; worthwhile to consider but not on the critical path to a clinical candidate. We had in hand the data package to convince us that riluzole was that clinical candidate, but we wanted as airtight a case as possible in flies.
Three months ago, we received a final report summarizing a battery of phenotypic rescue experiments that were performed focusing on riluzole in the close-out stage of the drug repurposing project with the Chow lab. Male and female flies were assessed in parallel. Significant p-values are displayed.
The original robust rescuable phenotype was muscle-specific-knockdown lethality. In this final round of validation studies, riluzole partially rescued muscle-knockdown lethality at 1 µM and 5 µM in both males and females. In absolute terms, riluzole doubles survival in both males and females in the muscle knockdown regime. There was no effect at 25 µM. These results are reassuring because they corroborate the results of the primary screen and the first round of hit validation. In other words, this is the third set of experiments that demonstrate a statistically significant rescue effect by riluzole.
Riluzole strongly rescues whole-animal knockdown lethality at 1 µM in males but has no effect on whole-animal knockdown lethality in females. In absolute terms, riluzole triples survival in the whole-body knockdown paradigm. What pharmacologists would call dose-limiting toxicity appears to preclude the rescue effect at 5 µM or 25 µM in either sex. It’s also possible that dose-limiting aversion is to blame, meaning the drug simply tastes bad to the flies.
FAM177A1 knockdown in glia causes seizures in a percentage of animals. Seizures are prevented at 1 µM in females, but oddly not in males. There was no effect at the two higher doses (5 µM and 25 µM) in either sex. We have no idea what is behind the male-specific and female-specific sex effects of FAM177A1 knockdown and rescuability. We also don’t know whether those sexually dimorphic phenotypes are a fly phenomenon or evolutionarily conserved in humans.
Seeing is believing, whether it’s a fly or a kiddo. This first video is of glial FAM177A1 knockdown in females. A handful of flies scurry up the wall immediately after shaking and inversion of the tube. But at least five animals are seizing and immobile on the bottom of the tube, eventually recovering and clambering up the wall but after a delay.
Now observe glial FAM177A1 knockdown female flies treated with 1 µM riluzole. None of the flies seized. Within 5 second of shaking and inversion the tube, all animals in the vial were scurrying up the wall or were already at the top.
The totality of the fly data consisted of reproducible and robust rescue of lethality in two sexes and in two knockdown regimes albeit with a dose-limiting ceiling. Riluzole has a maximum tolerated dose in people, too. The optimal dose that rescues lethality is the same dose (1 µM) that rescues a whole-animal brain-dependent phenotype like seizures as well as a functional outcome measure like wall climbing, which is the fly version of the 6-minute walk test. More accurately, the 6-second crawl test.
We had our eye on riluzole soon after the commencement of screening. Riluzole emerged as a 95% rescuer early in the drug repurposing screening campaign, but we waiting anxiously and patiently for the entire screen to be completed so we could contextualize riluzole against the backdrop of other hits. Through happenstance, we were able to be introduced to the CEO and CSO of Biohaven and presented the fly data package to them. They were incredibly receptive to the project and have been supportive ever since.
In the first six months of this year, Jill and Doug steadily built up conviction about giving riluzole to Charlotte. But they had to convince a clinician to become a champion too. They carefully diligenced what the starting dose and dosing schedule should be. The conditions finally came together over the summer, helped by the fact that their daughter Charlotte turned 18 and so is technically an adult from a pharmacokinetics and dosing perspective.
Here’s a before (baseline) video of Charlotte.
Here’s Charlotte after a week on the lowest dose of riluzole. Jill’s narration says it all.
Other improvements were noted in what turned out to be an initial honeymoon period. Unfortunately, a few weeks later Charlotte backslid to baseline. So far the riluzole journey has progressed in fits and starts. Immediately after a dose, Charlotte does great. But then the effect fades. After further consultation with her physician, a dose-escalation plan was implemented. Just last week, Charlotte reached the target dose of 200 mg/day. We excited and anxious to see how the next few months go.
Because science never sleeps, we’re collaborating with Unravel Biosciences and utilizing their rareSHIFT drug repurposing services platform. Charlotte will have a nasal swab RNAseq time course over several 48-hour intervals including during her dose escalation on riluzole. Meaning the levels of mRNA extracted from the epithelial cells in her nose will be measured resulting in a genome-wide gene expression profile snapshot. We’re excited for the potential of patient RNAseq as a N-of-1 pharmacodynamic (PD) marker. We might also consider running the SomaScan serum proteomics panel again to make a before riluzole vs after riluzole assessment.
This personalized drug repurposing and biomarker validation study opens the door to expanding riluzole (ideally ultimately troriluzole) to the other four FAM177A1 kiddos. Next on deck for riluzole is Jill’s and Doug’s youngest son and Charlotte’s younger brother, Cooper. But first we need to collect more observational data from Charlotte’s N-of-1, especially as she’s now ramped up to the maximum daily dose.
Given uncertainty about the FAM177A1 mouse model that so far has not yielded obviously actionable phenotypes, the FAM177A1 Research Fund is also hopping on the frog train to take advantage of a frog model of FAM177A1 deficiency. We have now flies, fish and frogs; all housed separately, of course. And fibroblasts and iPSCs at our disposal if necessary. The FAM177A1 disease model base is populating the FAM177A1 knowledge base, which is informing smart drug repurposing and preparing the ground for the modalities that come next.
After all, drug repurposing is a gateway drug: a springboard to targeted and curative therapies that build on the foundational successes of symptomatic treatments.