N-of-3 study of ibuprofen for MAN1B1-CDG
Dr Clement Chow's lab ran a fly-powered drug repurposing screen for MAN1B1-CDG. NSAIDs were the top hits. Three pioneer families self-organized an off-label observational study of ibuprofen aka Advil.
In collaboration with
Disclaimer
The results of the MAN1B1-CDG drug repurposing project that we are sharing in the spirit of open science below are novel preclinical research findings and therefore they do not constitute the practice of medicine.
Please consult your physician or clinical care team if you’re considering off-label use of any approved drug.
The same caution applies to nutraceuticals, supplements and “generally recognized as safe” compounds.
When a bolt of genetic lightning strikes a family and the smoke clears, a superpower is revealed. When genetic lightning struck their family, Claire and Matt mobilized on behalf of their son Jake. Claire’s and Matt’s shared superpower is a being a medical doctor by profession. As such, they’re in an ideal position to translate drug repurposing discoveries from patient avatars in the lab into 1-to-N medicine.
Physicians, heal thy child.
Holly Carmichael, cofounder of precious Maggie and Maggie’s Pearl, introduced me to Claire and Matt in early 2021. Over a series of Zoom calls, we devised a research plan with a fixed budget and contingencies. Little did we know that we’d have to invoke plan B several times. Claire and Matt found themselves in a succession of morale-draining research ruts lasting months, as often happens when improvising on the unexplored frontier of small-batch science.
Not all disease modeling expeditions are gently sloping straight lines. Complexity sneaks out of nowhere. We hit a wall with worms, and then got stuck in a cul-de-sac with fibroblasts. Flies were the last-ditch pivot a year into the project. Turned out more like flies to the rescue.
Professor Clement Chow’s lab at the University of Utah completed a fly-powered drug repurposing screen in summer 2022, and then followed up with hit validation studies in fall 2022. That’s where the MAN1B1-CDG Cure Odyssey left off and where we pick up the thread — or bar, as it were.
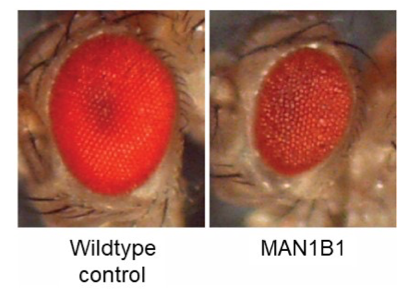
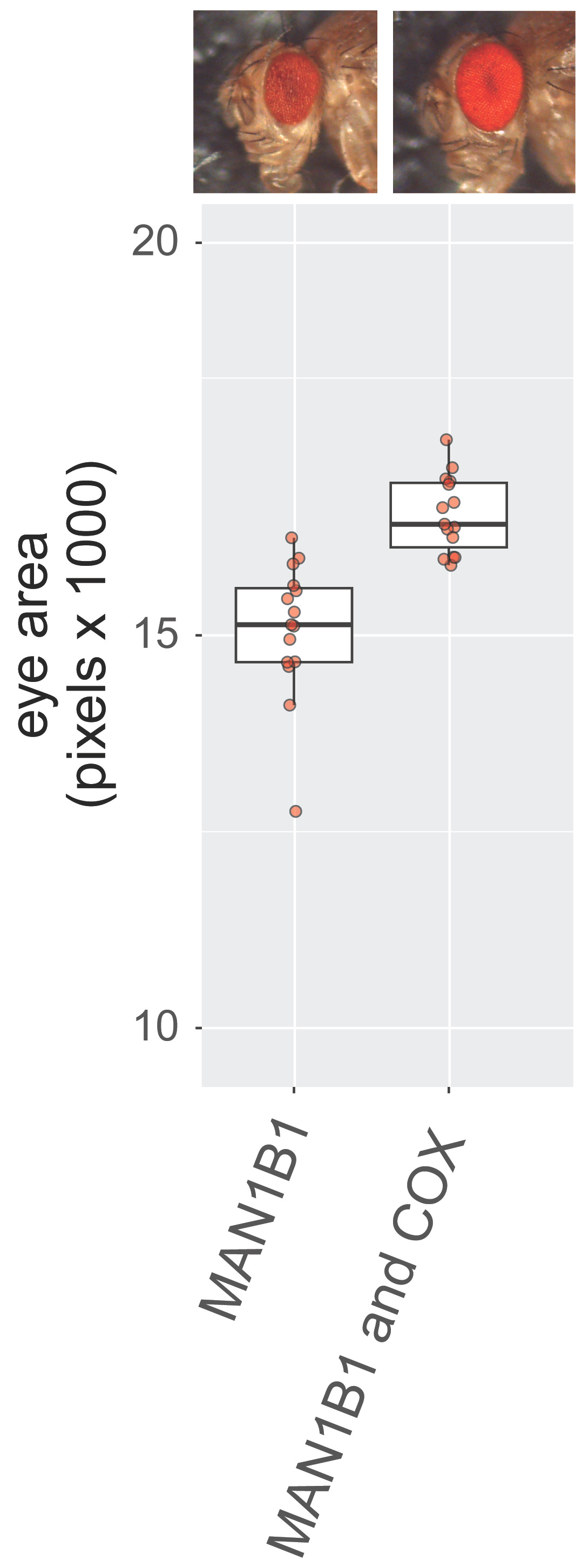
The Chow lab reduced the expression of the fly version of the MAN1B1 gene only in the cells comprising the fly eye. In cases where loss of a gene is not toxic, the fly eye develops normally. But if loss of a specific enzyme causes cytotoxicity in the fly eye, it results in what Drosophilists call a “rough eye” phenotype.
On the left, a healthy photogenic adult ready to explore the fruit bowls of the world. On the right, Rocky Balboa after a few rounds with Apollo Creed. 1,500 compounds that make up the Prestwick library were screened in the Chow lab, by hand, one vial at a time, over four months, in search of clinically actionable compounds that turn shriveled rough eyes into vibrant rubies.
Small-batch science paving the way for small-batch medicine.
The Chow lab’s MAN1B1-CDG fly screen yielded 54 rescuers with a Z score greater than 2. At the same time, the MAN1B1-CDG fly screen revealed 56 sensitizers with a Z score less than to -2. (By contrast, recall that the GMPPA-CDG fly screen, albeit a rescue of whole-animal lethality versus tissue-specific cytotoxicity, yielded only six rescuers). With so many rescuers from which to choose, we had to prioritize compounds by real-world clinical data, known pharmacology, and commercial availability.
In the course of poring over chemical structures, we noticed a peculiar pattern across the MAN1B1-CDG fly hits. There are repeated examples of structurally related pairs of compounds where one member of the couple is a rescuer but its partner is a sensitizer. Typically, structural analogs have the same effect in a screen, i.e., they’re either both rescuers or both sensitizers.
However, in the case of MAN1B1-CDG, the pattern of a pair of structural analogs that have opposite effects is evident across a dozen pharmacological classes that include: steroids, catecholamine action, antibiotics, local anesthetics, alpha adrenergics, beta adrenergics, diuretics, and sulfonylurea anti-diabetics. The dose makes the poison? Can there be too much potency, pharmacologically speaking?
As a first example, consider a tale of two glucocorticoids. Fluticasone is a MAN1B1 rescuer (Z score = 2.31) but its doppelgänger clobetasol is a MAN1B1 sensitizer (Z score = -2.45).
Across 20 drug repurposing programs spun up by Perlara and pioneer families since 2021, closely related hit compounds with the same mechanism of action usually have the same effect in a drug repurposing screen. We’ve only occasionally seen the contrarian pattern where chemical cousins have opposite effects, most of these instances are congenital disorders of glycosylation like MAN1B1-CDG.
Here’s a second duo of divergent doppelgängers. The atypical antipsychotic risperidone is a MAN1B1 rescuer (Z score = 2.55), but its pal pirenperone is a MAN1B1 sensitizer (Z score = -2.24).
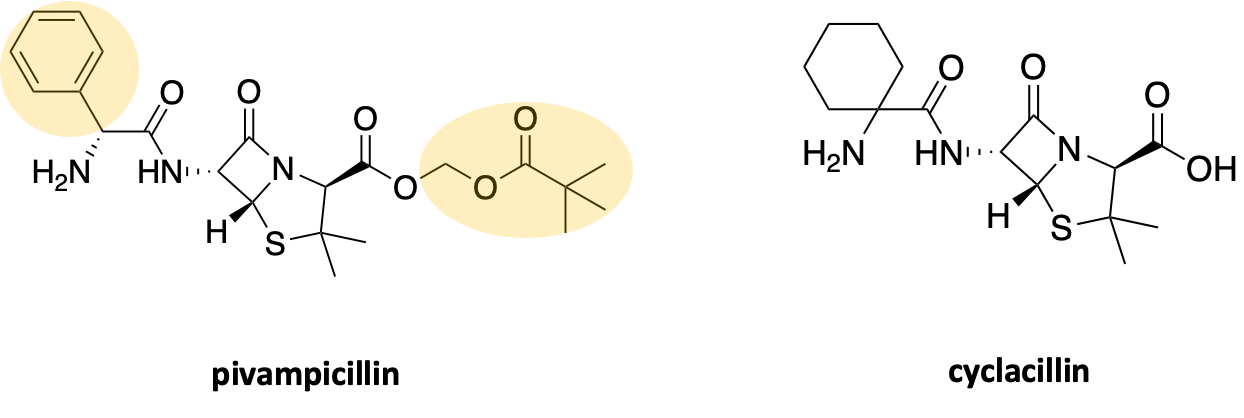
A third illustration is a pair of penicillin derivatives. Pivampicillin is a MAN1B1 rescuer (Z score = 4.09). A few cosmetic adjustments leads to cyclacillin, a MAN1B1 sensitizer (Z score = -2.41).
Not to belabor the point, but here’s a fourth example involving a pair of beta adrenergic antagonists. You know the drill: atenolol is the MAN1B1-CDG rescuer, bisoprolol is the MAN1B1-CDG sensitizer.
It gets really interesting when we examine the largest pharmacological class of MAN1B1-CDG rescuers. None other than some of the most widely used medicines on the planet: nonsteroidal anti-inflammatory drugs, or NSAIDs.
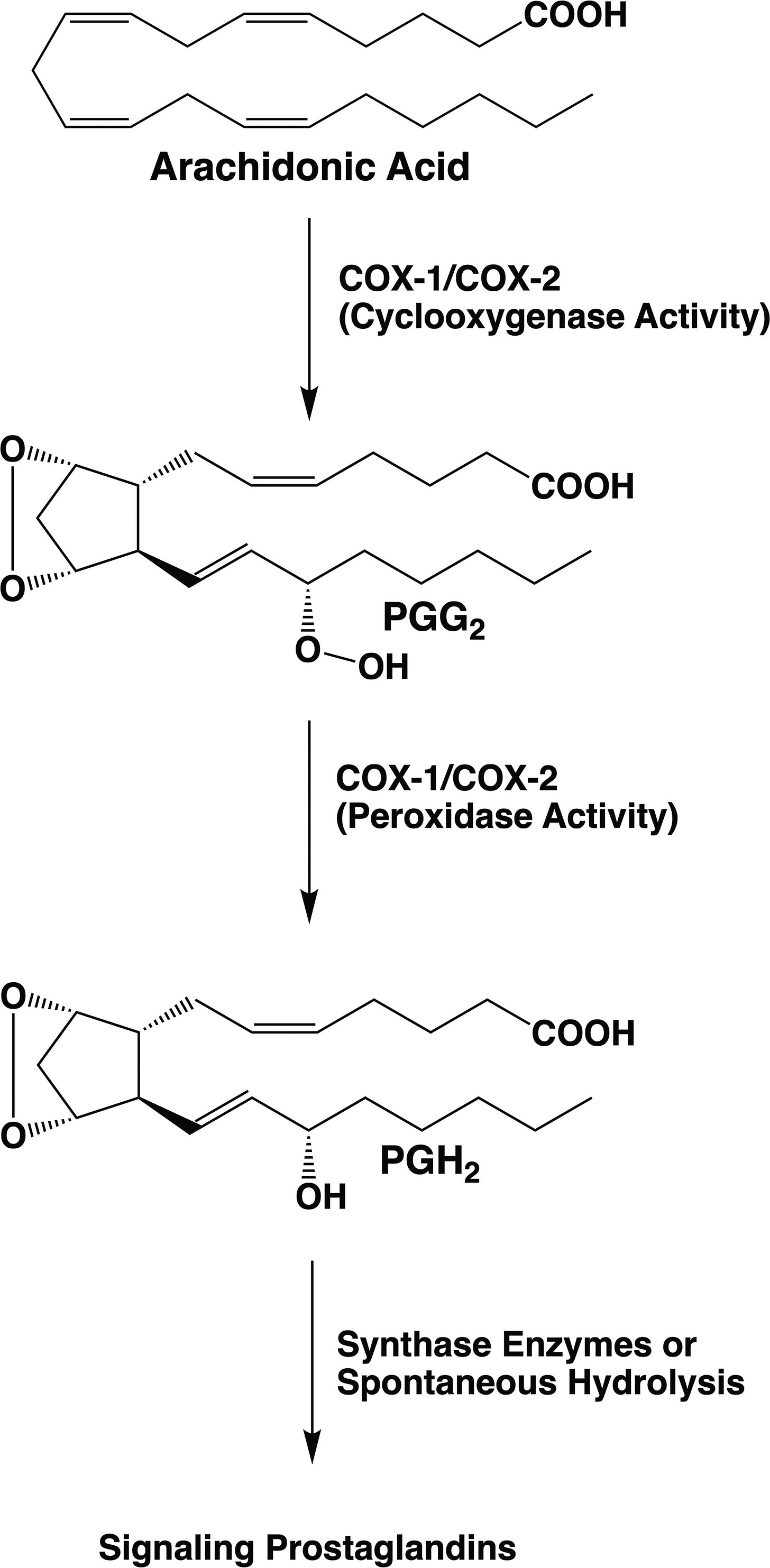
NSAIDs target cyclooxygenase 1 (COX-1) and cyclooxygenase 2 (COX-2) enzymes, which convert the lipid arachidonic acid into drivers of inflammation called prostaglandins. COX-1 is constitutively active and has a long protein half-life, whereas COX-2 is inducible and has a short protein half-life. The alias of COX-1 and COX-2 is prostaglandin synthase (PGSH). The fly genome encodes a single cyclooxygenase isoform called COX.
9/54 (16%) MAN1B1-CDG fly rescuers are NSAIDs. Two MAN1B1-CDG fly sensitizers are also NSAIDs, indicating a dose-limiting toxicity that will be discussed in greater detail. The fly screen also revealed five rescuers with anti-inflammatory properties, and two prostaglandin-like rescuers, which act as competitive inhibitors of COX-1 and COX-2.
It was a completely unexpected result. A reminder why we do unbiased drug repurposing using patient avatars in the first place. The most exciting part was the clinical actionability. A bottle of ibuprofen is sitting in everyone’s medicine cabinet. No need for an IRB or spIND. Although chronic long-term use is not an option, ibuprofen is demonstrably one of the safest medicines on the planet. Also on the Essential Medicines List published by the World Health Organization.
Here are the chemical structures of the nine NSAID rescuers.
Phenybutazone and feprazone are chemical twins separated at birth, the pattern we expect to see. Ibuprofen, like most of the NSAIDs shown above, is a nonselective COX inhibitor. Phenacetin approval was revoked by FDA. Fortunately, we could swap it out for acetaminophen.
Three COX-2 selective NSAIDs were included in the hit validation phase: celecoxib (Celebrex), rofecoxib, and the structurally distinct lumiracoxib, which was actually a sensitizer in the primary screen.
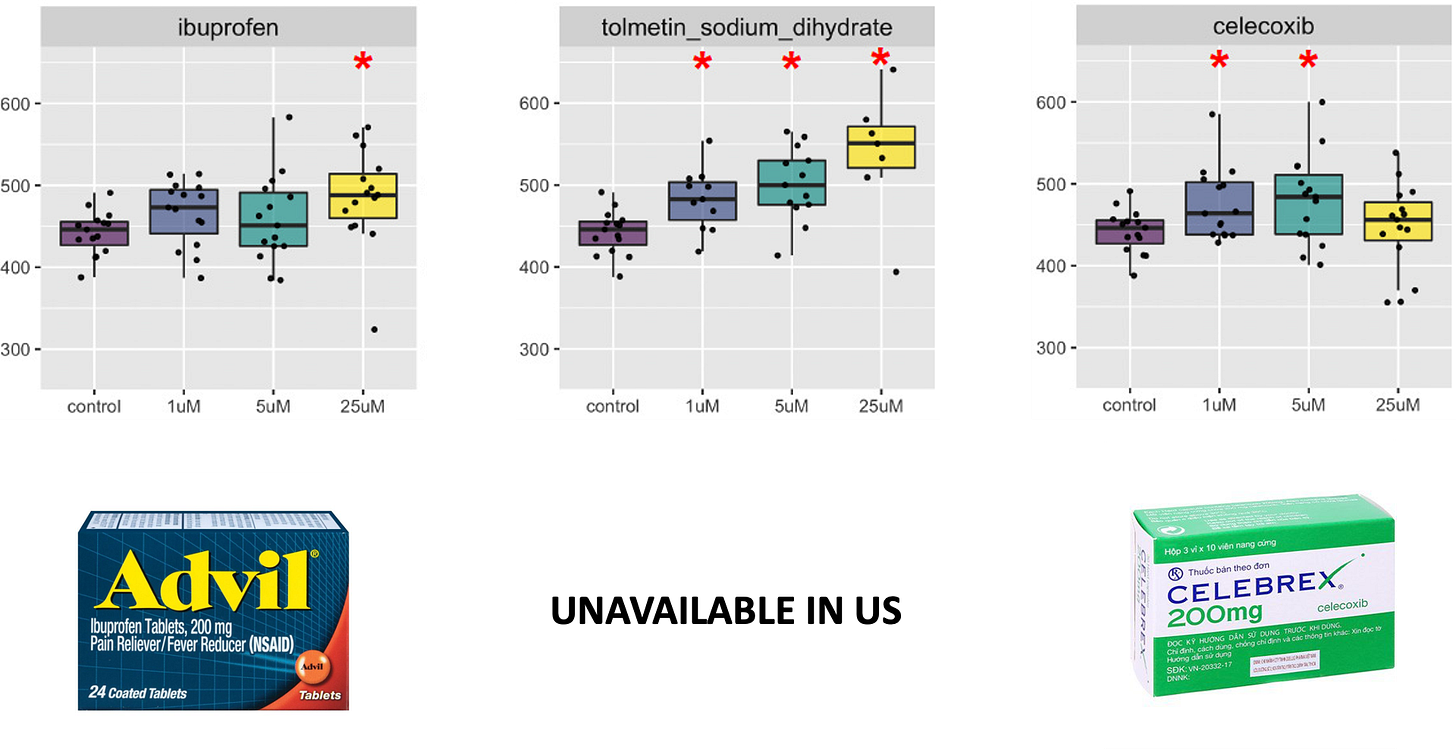
16 compounds, mostly NSAIDs, were evaluated in rough eye rescue dose response experiments performed in the Chow lab. Two compounds were not soluble enough in fly food to work with, so 14 hits were actually validated, as summarized in the multi-panel figure below.
With the sole exception of 4-aminosalicylic acid, the 13 other compounds all had at least one dose that resulted in a statistically significant rescue of the rough eye phenotype (red asterisks). Tolmetin produced the cleanest dose response, but no generics company currently manufacturers a US supply. Ibuprofen has a smaller effect size relative to other NSAIDs but importantly lacks any dose-limiting toxicity observed at 25 µM celecoxib.
Overall, 7 of the 14 compounds exhibit statistically significant rescue at all three doses tested. Interestingly, all three COX-2 selective NSAIDs exhibit dose-limiting toxicity. For celecoxib and lumiracoxib, the peak rescue occurs at 5 µM. For rofecoxib, the peak rescue occurs at 1 µM.
Is the fly telling us that we want to inhibit COX-1 and COX-2 equally, or just weakly? Or is it okay to hit COX-2 selectively using a potent inhibitor but we need intermittent versus continuous dosing to avoid on-target toxicity? At this time, all we can do is make educated guesses based on our data and published data.
NSAIDs target the active site of the enzymes COX-1 and COX-2, both of which are cyclooxygenases that feed on a metabolic substrate called arachidonic acid.
As recounted in Pharma Chameleon, a shape-shifting pharmacophore has emerged from nearly every Perlara drug repurposing hit list. NSAIDs are echoes of arachidonic acid and its high-energy transition states that form in the active site of COX-1 and COX-2. The arachidonic acid pharmacophore coil ups like a cobra into the twin benzene rings of phenylbutazone and celecoxib. Rosuvastatin is a curious hybrid of a NSAID and a statin, structurally speaking.
Can we connect the dots between MAN1B1 and NSAIDs? A PubMed search reveals tantalizing clues. A 2006 paper (Mbonye et al., 2006) directly tied MAN1B1 and COX-2 together via a process called endoplasmic reticulum associated degradation (ERAD), pinpointing glycosylation of asparagine 594 as indispensable for the degradation of the COX-2 enzyme. MAN1B1 initiates a glycan trimming process that sends COX-2 on its way to the cellular trash bin. Meaning once the inflammatory trigger subsides, COX-2 protein is meant to be rapidly cleared from the cell.
Another paper (Singh et al., 2012) showed that decreasing MAN1B1 protein levels led to an increase in COX-2 protein levels. Perhaps loss of MAN1B1 disturbs glycosylation of COX-2 and its naturally fast turnover? So instead of MAN1B1 being an indiscriminate housekeeping enzyme that clips of mannose resides willy nilly from any glycoprotein, MAN1B1 specifically regulates the glycosylation of particular client proteins, in this case COX-2.
A similarly counterintuitive pathomechanism has emerged for another CDG: NGLY1 deficiency. NGLY1, which encodes an N-glycanase, ostensibly has cellular housekeeping functions that affect the deglycosylation of all glycoproteins. But all published evidence from disease models like worms and flies and human cells paints the opposite picture. NGLY1 regulates the activity of a handful of critically important client proteins like NRF1, BMP and NKCC1 using deglycosylation of specific asparagines as a form of post-translational regulation.
If something analogous is happening between MAN1B1 and COX-2, what would happen if a kiddo with MAN1B1-CDG was treated with a NSAID?
Innocent until proven guilty. That’s the guiding principle of our legal system. Safe until proven dangerous. That should be the comparable guiding principle of our medical system. Especially small-batch medicine where the standard of care is nothing because there is no standard of care for 95% of the 10,000+ rare diseases.
Of all the NSAIDs, ibuprofen seemed the most reasonable to try. Remember the old McDonald’s tag line: Billions served. Ibuprofen has been taken by over billion people at this point in its 62-year history. That safety record speaks for itself. But what about more convincing evidence that would predict treatment effect on the autism symptoms of MAN1B1-CDG?
One experiment we could only do in the lab versus a human was to knock down COX in flies in which MAN1B1 is also knocked down to see if loss of COX enzymatic activity rescues the rough eye phenotype. Two genetic wrongs make a phenotypic right. In other words, is genetically knocking down COX in flies equivalent to pharmacological treatment of flies with ibuprofen?
The answer is: yes! This result suggests the rescue effect of ibuprofen is caused by inhibition of COX. In the fly, we infer that loss of the MAN1B1 enzyme somehow leads to an increase in COX enzyme activity, which can be pharmacologically relieved by a NSAID like ibuprofen, or genetically relieved by not having COX in the first place.
The primary screen and the hit validation studies we just presented all use the fly rough eye model. One could reasonably argue for evidence of rescue of a neurological phenotype in flies. Turns out when MAN1B1 is knocked down in the fly brain versus the fly eye, there’s a strong “bang sensitivity” phenotype as shown in the two videos below.
Bang sensitivity is when you literally bang a vial full of flies on the benchtop, thrusting them all downwards. Normal flies hastily start climbing up the walls of vial. MAN1B1-brain-knockdown flies don’t recover the bang and remain dazed or have seizures at the bottom of the vial.
Here’s what happens at 1 µM ibuprofen, which is below the effective dose and comparable to the no-drug control. This video (and the next one) start right after the bang. A few flies attempt to scale the sides but fall down immediately. None of the flies make it off the bottom.
By contrast, here’s what happens at 50 µM ibuprofen, which is a bioactive, non-toxic dose. You’ll observe that about half of the flies are climbers.
Clearly, ibuprofen has a rescue effect on a brain-based phenotype, not just rescue of eye cell proliferation and survival in what could be argued is an artifactual physiological setting. But would ibuprofen have any positive effects on a child living with MAN1B1-CDG?
Based on the totality of evidence summarized above, Claire and Matt decided to move forward with ibuprofen. A pioneer observational study with Jake a little over one year ago on November 15, 2022. Claire and Matt consulted with CDG expert Dr Eva Morava a few weeks later about how long to dose for and what additional in vitro tests could be done on MAN1B1-CDG patient fibroblasts to test the NSAID hypothesis.
Most of the data are diary entries and other observational notes, even text messages from group chats. Some clinical trial traditionalists will dismiss any bottom-up, parent-led effort as riddled with bias or unreliable data. Those who are more charitable about the concept of small-batch medicine might still reasonably insist on knowing what outcomes will be measured to demonstrate treatment effect. This is why they’re called observational studies. If rare disease parents are really good at one thing it’s observing their kids.
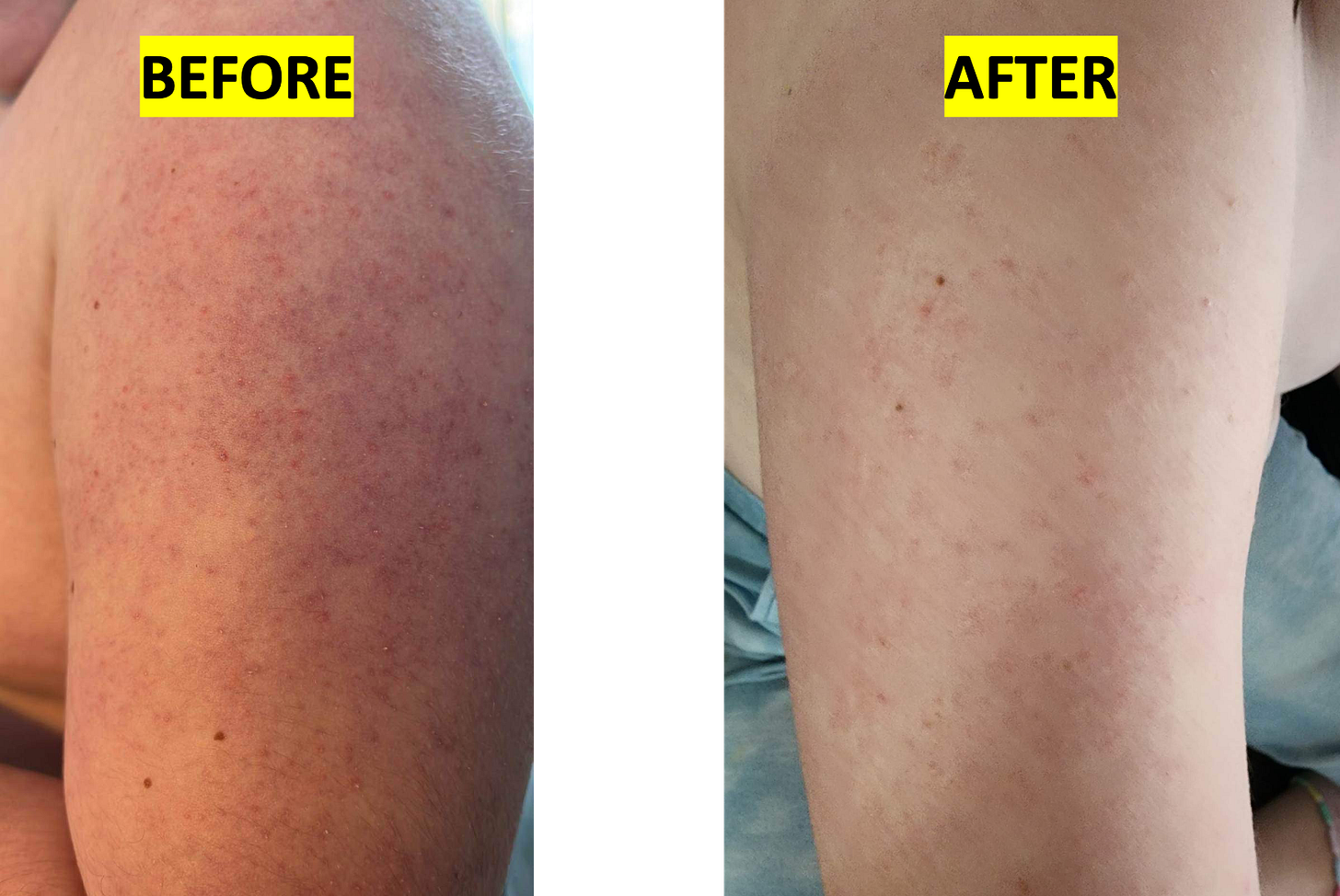
To wit, an unexpected skin biomarker revealed itself in the course of Jake’s pioneer observational study. Keratosis pilaris (KP), a relatively common skin condition colloquially referred to as chicken skin, is a common symptom in MAN1B1-CDG. Within two weeks of taking ibuprofen, Jake’s KP resolved. It’s hard to tell because of the swelling and redness caused by a bug bite, but the bumps characteristic of KP have all but vanished in the pictures below.
But then the KP started creeping back by Week 10, as shown below. It had fully returned to the pre-ibuprofen baseline by Week 14. Claire noted in the summary spreadsheet: “We noticed the KP had slightly returned after decreasing from tid to bid dosing.”
What about cognitive or neurological changes? With respect to sleep, Claire observed:
Waking 30 minutes earlier as soon as we started, waking at 5:15. This fluctuated when he got influenza but now he's back to waking at 5:15. We thought the bid dosing improved his sleep but I think that may have been more related to the influenza.
Other parent observations from Claire and Matt:
more interested in engaging with people, even people he is meeting for the first time (grabs their arm to get their attention and smiles at them)
communicating more: pointing to things, now consistently saying 'no', trying to say 'off' and 'on'
now able to write all his lower case letters independently (previously required support at the wrist to guide his writing)
more relaxed: willing to try new foods, go to new places (ski lessons), be at a crowded party without issue
In terms of weight, MAN1B1-CDG is unlike other CDGs. Weight gain is an issue, whereas in PMM2-CDG and most other CDGs the kiddos knock back several PediaSures per day but are still underweight. Claire and Matt noticed that Jake’s weight did not change during his two months on ibuprofen. After four weeks off ibuprofen, Jake gained 2.5 pounds.
Small-batch medicine is another way of saying 1-to-N medicine. In this case, from 1 to 3. Two more North American MAN1B1-CDG kiddos — Remi in the US and the Olivia in Canada — started their ibuprofen observational studies three and four weeks after Jake started his study, respectively. No clinical trial coordinator. No clinical trial overhead. In retrospect, a geographically distributed, staggered start, open label, Phase 1/2 study design. Lean startup meets precision medicine.
Olivia’s response to ibuprofen is similar to Jake’s treatment response along multiple axes. First, her KP resolved in the first week of treatment. However, by the third week it was back in full force if not a little worse by the fourth week. What was causing this rebound effect, evident in 2/2 kiddos?
For Olivia, we have four weeks of data. Like Jake, Olivia’s weight stayed constant during ibuprofen treatment. In fact, she lost half a pound while on ibuprofen. However, she gained 15 pounds four weeks after going off ibuprofen.
In terms of any neurological, cognitive or behavioral improvements, the second week on ibuprofen Olivia’s parents noted that she was not as “whiny for food" and “it seems that she is maturing a lot.” Caretakers and teachers noted improvements during the fourth week of ibuprofen treatment. The same early riser lark effect seen with Jake was noted with Olivia in the third week on ibuprofen.
At roughly the same time that Olivia started on ibuprofen, Remi did too, becoming the third pioneer MAN1B1-CDG kiddo to try ibuprofen. Remi appears to be the hyper-responder among the trio of MAN1B1-CDG pioneers. She is also the least affected of the three kiddos. The convergence on the same changes, many of which can be objectively called improvements, across three out of three pioneer MAN1B1 kiddos is noteworthy.
First, Remi’s KP improved in Week 1 but was returning to baseline by Week 2, and then remained unchanged for the rest of the 6-week observational study. Second, Remi’s weight held basically flat over the 6-week treatment period, but then she gained 2.5 pounds four weeks after stopping ibuprofen.
Third, here’s a compilation of text and video updates from Remi’s mom which capture the communications and cognitive gains. Note that Remy was not doing any adding before ibuprofen:
Remi’s elementary school teacher offered the following real-world evidence:
It was agreed that after 6 weeks on drug, and then the kiddos would go 4-6 weeks off drug to see if treatment effects were lost. Ibuprofen is not prescribed for chronic use. When all three kiddos went off drug, they eventually regressed and lost some or all of their gains, an indication that the drug was responsible for the effect.
Were the gains seen in all three kiddos real or just coincidental spontaneous developmental spurts that are sometimes seen in the natural history of CDG? Or should this be viewed from the lens of variable treatment response where one out of the three kiddos exhibited remarkable communication and cognitive gains on ibuprofen?
This past spring, Dr Morava’s lab generously tested COX-1 enzymatic activity and COX-2 enzymatic activity in four MAN1B1 fibroblast lines. Frustratingly, the results are not consistent with the results of the MAN1B1-CDG fly models.
What happens when disease models disagree? Which one overrules? If we were testing COX-1 and COX-2 activity in neurons or glia instead of fibroblasts, would we see concordance with the fly data package? We are left with more questions than answers at this juncture.
The convergent flickers of efficacy in the ibuprofen n-of-3 parent-led observational study leave us wanting more but the risk of rebound and regression gives us pause. MAN1B1 turns out not to be an easy gene. Loss of MAN1B1 can be shrugged off by most cells but some cells, in particular neurons and/or glia are particularly sensitive. If any researcher reading this wants to help figure out why, by all means join the MAN1B1-CDG cause.
With NSAIDs on pause, what about rosuvastatin (Z score = 2.75) or guaicol (Z score = 4.68), which is actually chemically similar to vanilla? Both rosuvastatin and guaicol validated in the 3-point dose response study alongside ibuprofen. It is worth reconsidering those but also keep in mind every intervention is a several month commitment if it’s to be done right and produce meaningful data. Risperidone showed a nice dose response in the hit validation study. The concern though is the weight gain that is a known side effect of antipsychotics like risperidone. MAN1B1-CDG patients already struggle with hyperphagia and weight gain.
In parallel, a new dawn rises. More recently diagnosed MAN1B1-CDG families are taking up the drug repurposing and disease modeling torches where Claire and Matt have left off. Cure odysseys are ultramarathons. One family can’t be expected to go the distance by themselves. 1,500 compounds were screened in the first drug repurposing campaign. The goal now is to screen at least 4,000 compounds in a fly model, ideally using the brain-specific MAN1B1 knockdown model and screening for compounds that rescue a neurological phenotype.
Onward for MAN1B1-CDG in 2024! Jake, Olivia, Remi and other kiddos like them are counting on it.





















Amazing work from everyone, Ethan. And I am always engaged by your writing style, and your transparency.