Restoring balance to the flux
CDGs are a 200+ gene family of inherited metabolic diseases, GMPPA-CDG and SRD5A3-CDG to name a few. Turns out GMPPA deficiency is rescued by SRD5A3 deficiency 🤯. Two genetic wrongs can make a right.
In collaboration with
Disclaimer
The results of the GMPPA-CDG drug repurposing project that we are sharing in the spirit of open science below are novel preclinical research findings and therefore they do not constitute the practice of medicine. Please consult a physician or clinical care team if considering off-label use of any approved drug or compassionate use of any experimental drug. The same caution applies to nutraceuticals, supplements and “generally recognized as safe” compounds.
For most rare genetic diseases, cure-focused research depends entirely on entrepreneurial families seared by a bolt of genetic lightning. The reverberations thunder into reality when the first life-shattering signs of disease manifest. Often at birth but sometimes lurking for years before the shockwaves suddenly make impact on the fateful day a confirmed diagnosis is delivered in a genetic test report.
A cruel stroke of the genetic pen converts the book of life into its opposite. Well-made plans go out the window. Dear readers of Cure Odysseys are by now all too familiar with the unrelenting tragedy of rare genetic diseases that unfolds mercilessly every day across the globe. And so they also know all too well that tragedy fuels triumph.
Not all parents are in a position to buck the advice of credentialed experts and bushwhack through the wild lands of basic science, translational research, and clinical trials. But some parents can’t help themselves. Through grit, self-instruction and mentorship, founder parents reveal their inner scientist, community builder, and drug developer — oh and toss fundraiser in the mix, too. Traits they might not have manifested in their professional lives before their child’s diagnosis. Or never even seen in themselves before.
No sane parent signs up for the rare disease life. They are wickedly thrust into the arena, like a righteous gladiator. The collective force of will of a community of affected families organized by its natural leaders makes rare disease medicines possible. Spinal muscular atrophy (SMA), Angelman syndrome, cystic fibrosis are just three top-of-mind scientific and commercial success stories in rare.
But a well-versed critic is fair to point out that those disease are common to medium rare. What about the long tail of rare genetic diseases affecting a few dozen to a few hundred patients? It’s lonely out there.
The only consolation of a catastrophic genetic lightning strike is that it mobilizes exceptional founder parents to put their children’s rare genetic disease on the map, and then devise a plan to wipe it from the face of the Earth.
CDGs — Congenital Disorders of Glycosylation — are a natural experiment in what happens when families are nucleated into a community by one or more founder parents. From the PIGN Cure Collective and Cure DHDDS on the larger side to Behind the Rainbow (PIGW-CDG) and Cure SRD5A3-CDG on the smaller side, we’ve seen these community nucleation events across CDG subtypes over and over again, and across the rare disease landscape for that matter.
We obviously wouldn’t be talking about GMPPA-CDG if there weren’t a founder parent whose child is personally affected. That parent is Diana Roetting and her daughter Rosie, who is living with the particularly uncommon CDG subtype called GMPPA-CDG. Diana knows of just six GMPPA-CDG patients total around the world, one of the rarest of the rare genetic diseases.
This story is about how one family is creating a scalable cure blueprint for a disease so tiny you can count the patient population on two hands.
When we last checked in on the GMMPA-CDG cure oddyssey two years ago, we had evaluated and then ruled out a GMPPA-CDG worm model. As a hedge, we pursued a fly model in parallel. We were handsomely rewarded by a strong lethality phenotype, meaning any rescuer compound would have to overcome the high bar of lethality and propel affected embryos to adulthood.
CDGs are a family of inherited metabolic diseases. Nearly 200 genes encode a protein that serves as a node in the cellular sugar supply chain. Sugars are imported as raw materials, processed into precursors and advanced parts, assembled into manufactured goods, and exported as finished products. A robust sugar supply chain keeps the cell humming.
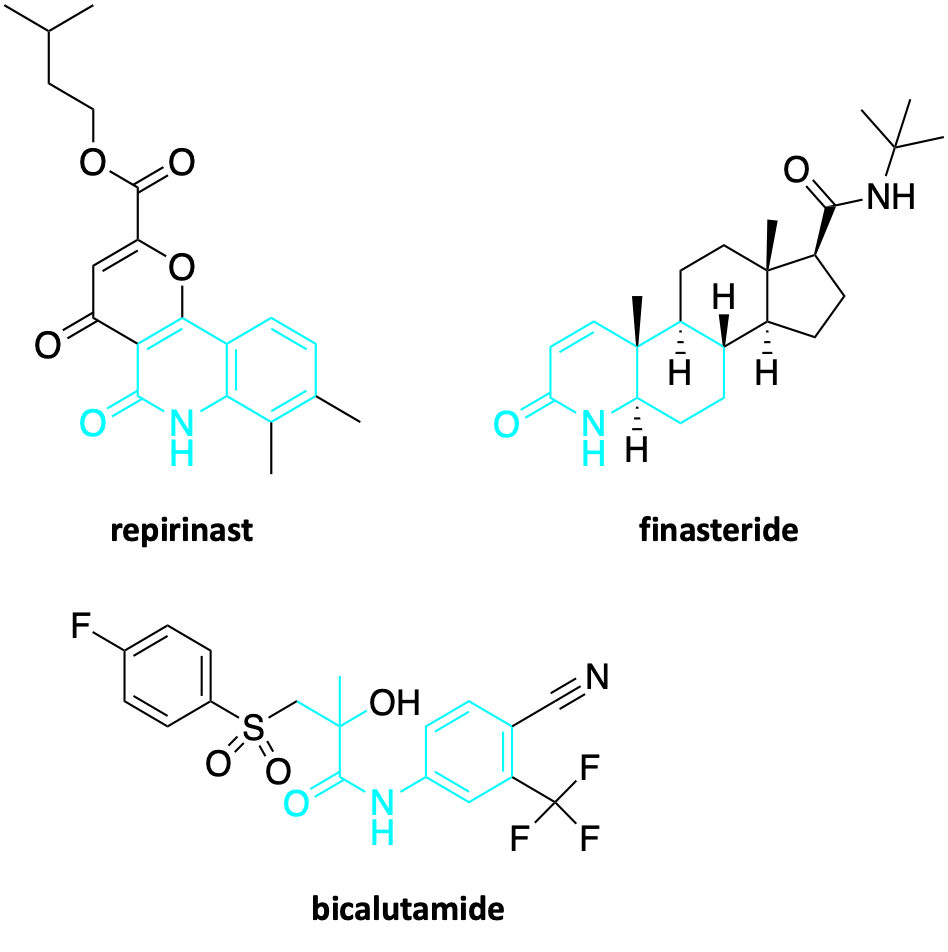
The GMPPA enzyme is specifically involved in generating the precursors of the glycan chains that get snapped onto proteins at specific amino acid attachment points, e.g., asparagine residues. The enzyme converts mannose-1-phosphate to GDP-mannose, a so called activated nucleoside sugar. Imagine Lego bricks that can be stacked into towers.
GMPPA has a dancer partner called GMPPB, so the pathomechanism is more intricate. Loss-of-function mutations in the GMPPA gene leads to overactive GMPPB protein activity. In other words, the loss of GMPPA function leads to a gain of GMMPB function, ultimately resulting in excess glycosylation, specifically hypermannosylation. Normally in CDG, the disease driver is insufficient glycosylation, or hypoglycosylation. But in the case of GMPPA-CDG, too little of something but also too much of something — in this case GDP-mannose precursor — can be a bad thing according to the still inscrutable wiles of the wire diagram of metabolism.
From a cellular biomanufacturing point of view, glycan chains have extremely tight specs. An extra mannose here, an unexpected branch point there, and soon enough the entire proteome is riddled with defective or suboptimal glycans. Some proteins and pathways are more vulnerable to glycosylation imbalance than others, a recurring theme across inherited metabolic diseases. This heterogeneity bubbles up as a spectrum of symptoms and symptom severity in patients living with GMPPA-CDG.
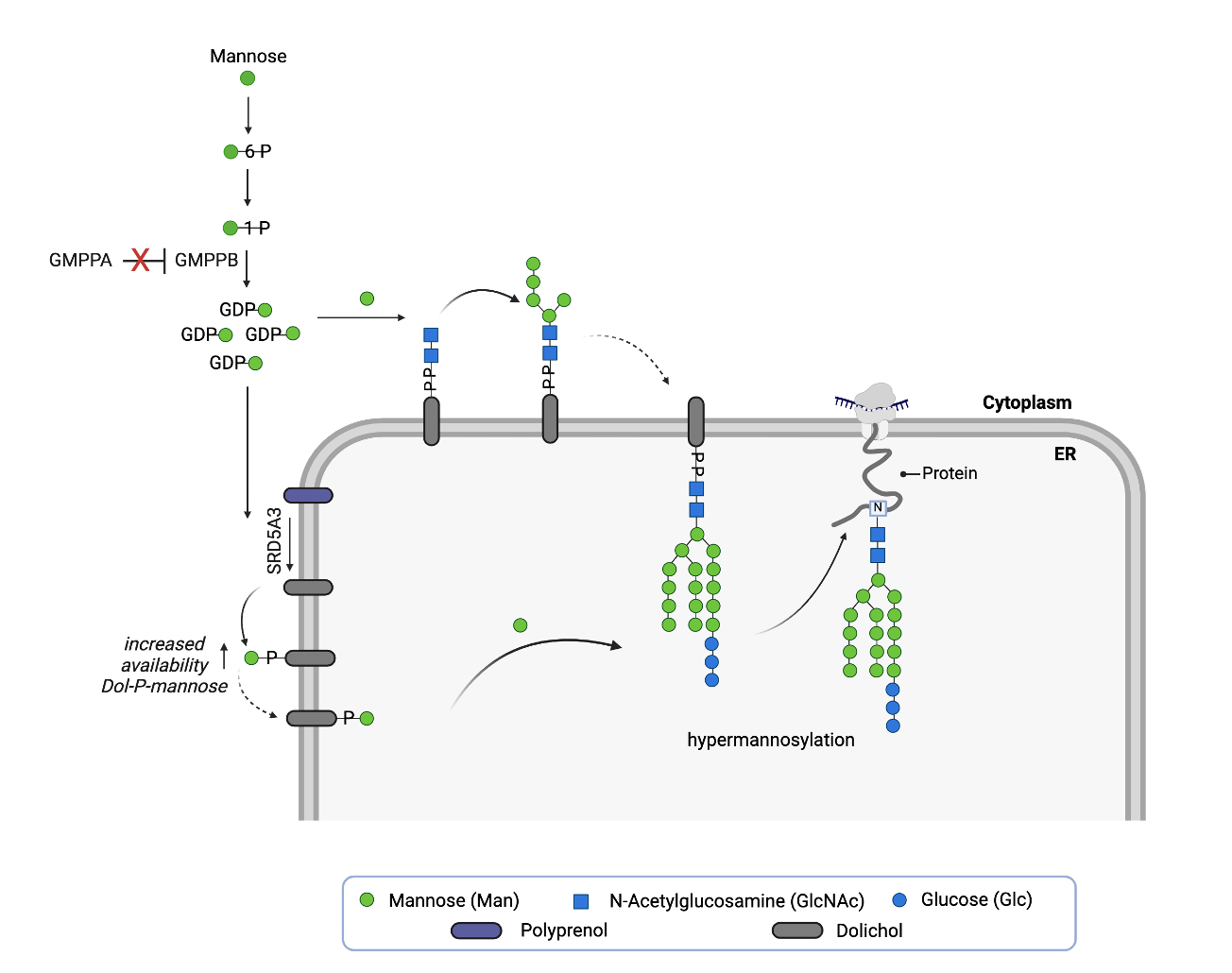
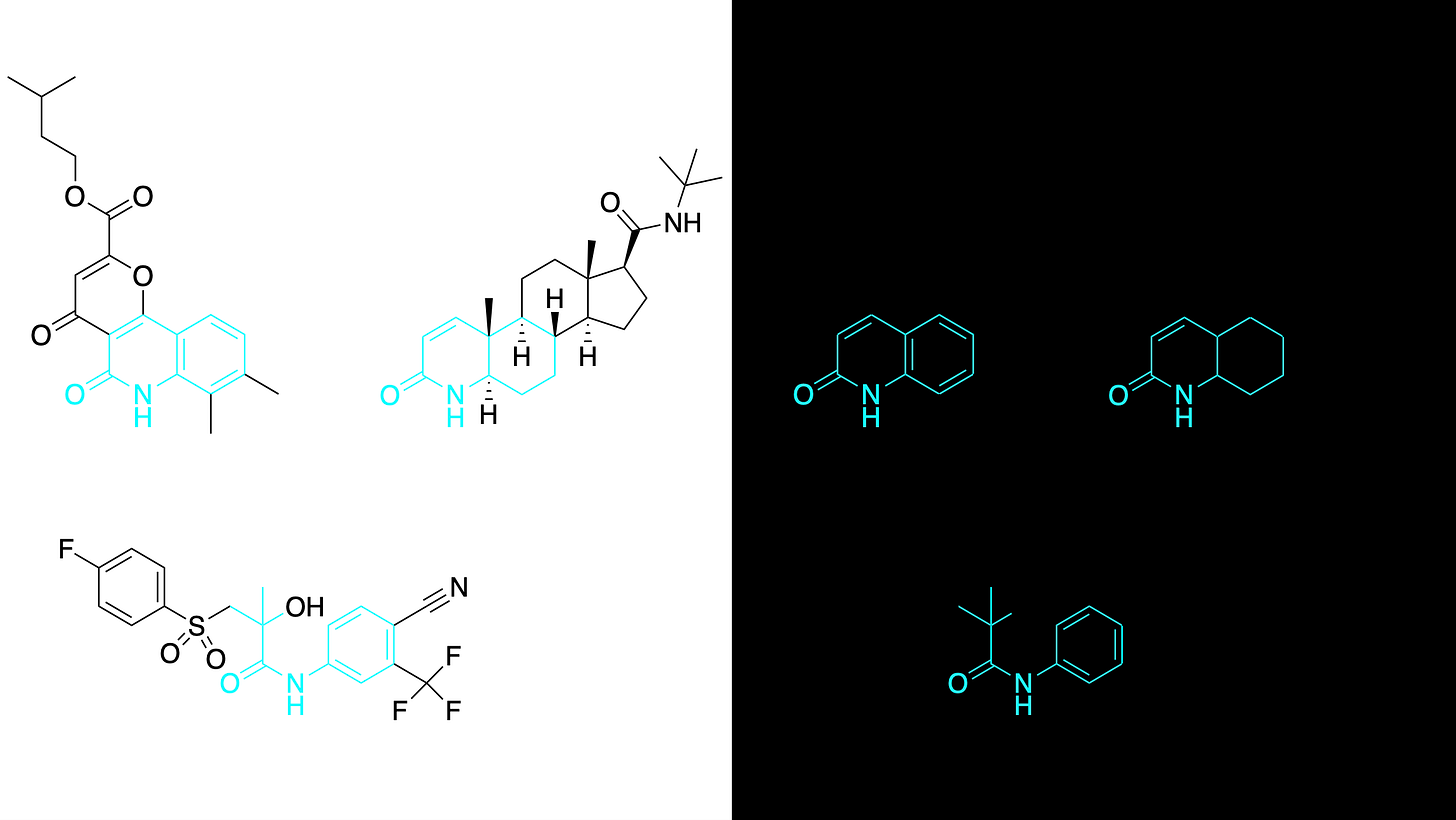
Two years ago, a fly screen of over 1,000 known drugs in Professor Clement Chow’s lab at the University of Utah surfaced three top hits that rescue lethality of GMPPA deficient flies, and, remarkably, share a hidden pharmacophore: repirinast, finasteride and bicalutamide.
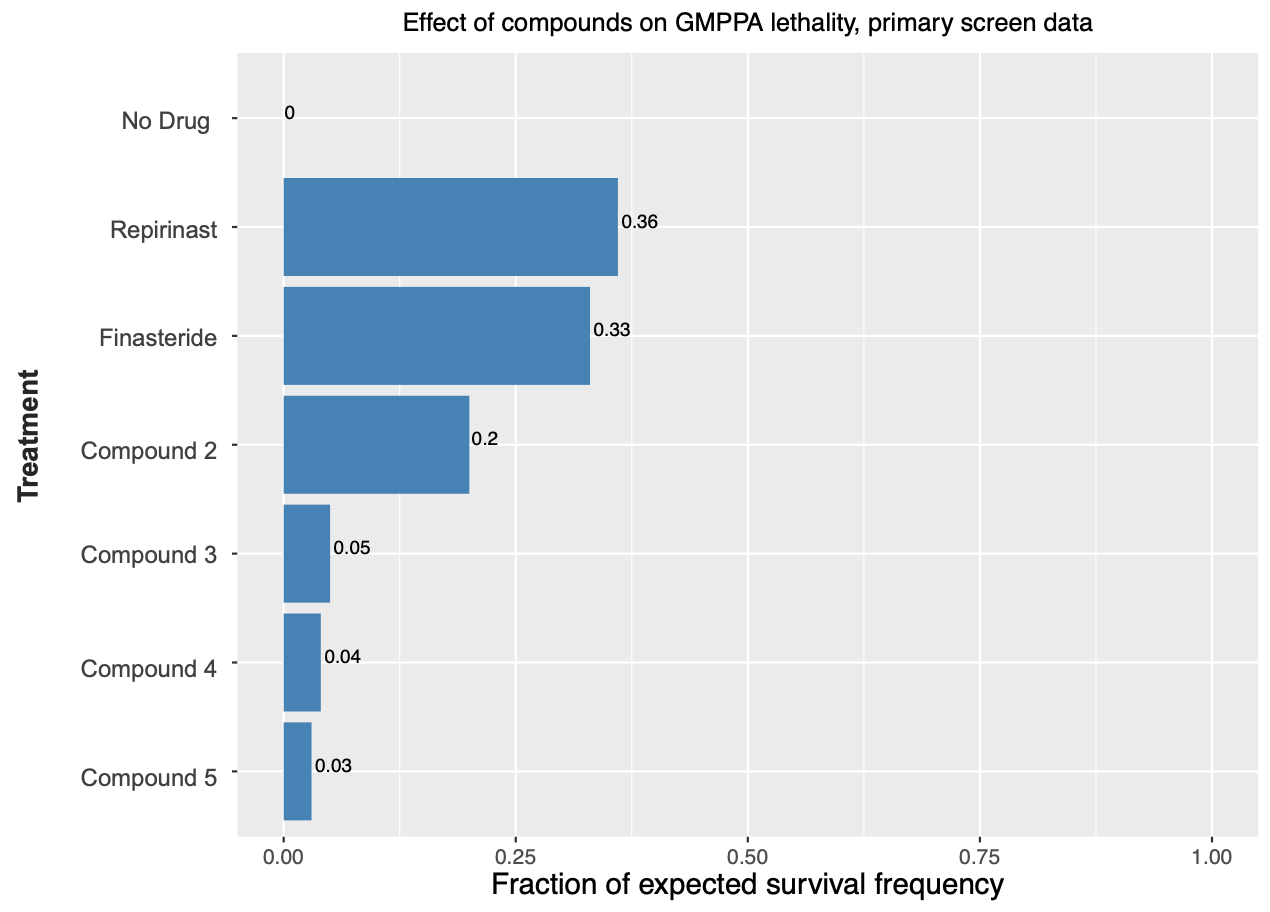
Repirinast is a mast cell inhibitor and antihistamine developed for the Japanese market in the 1970s and 1980s, but has since been discontinued for commercial reasons. The known on-target of repirinast did not immediately present a connection to GMMPA or glycosylation. So what do these three compounds have in common? The breakthrough insight was realizing that finasteride targets SRD5A2, an enzyme closely related to SRD5A3, which was first described as a CDG disease-driver gene in 2010.
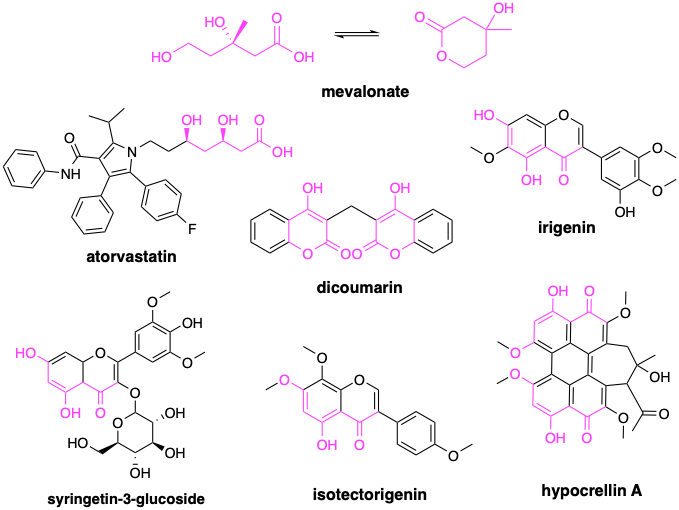
With the proper lens to refract the data, a clear mechanistic hypothesis emerges from the three hit structures in isolation, denoting a conserved structure-activity relationship, or what we refer to as the SRD5A3 active site pharmacophore, which is depicted below in cyan. Independent of chemical structure, the known shared pharmacology of the steroidal antiandrogen finasteride and the nonsteroidal antiandrogen bicalutamide further corroborates the prediction: repirinast is a cryptic SRD5A3 inhibitor.
We’ve revealed pharmacophores like this in almost every drug repurposing screen — now numbering over a dozen examples — regardless of which flavor of patient avatar is used to conduct the screen: yeast, worm, fibroblast, or in this case, fly. We’ve also seen pharmacophores in drug repurposing hit lists from Unravel Bioscience, which skips the patient avatar step and samples gene expression in patient cells from a nasal swab.
The SRD5A3 pharmacophore is observed in both the closed conformation, as with repirinast and finasteride, as well as in the open conformation of bicalutamide. That’s another pattern we’ve seen before: cyclic versus linear versions of the same pharmacophore. Coincidentally, it just so happens that we observed that same pattern in the worm-powered screen for SRD5A3-CDG, where the mevalonate pharmacophore (in purple) reveals itself, or a fragment thereof, in both linear and cyclic forms in the top hits from the screen.
Does the link between GMPPA-CDG and SRD5A3-CDG go deeper? You bet it does.
Based on the totality of the data collected thus far, we hypothesize that SRD5A3 inhibition is a rescue mechanism that compensates for the effects of GMPPA deficiency. Rebalancing glycosylation in the context of GMPPA deficiency means somehow compensating for or relieving the surfeit of GDP-mannose flooding into the sugar supply chain upstream of SRD5A3. Rebalancing metabolism is another recurrent theme: if one dial is throttled up, another is turned down.
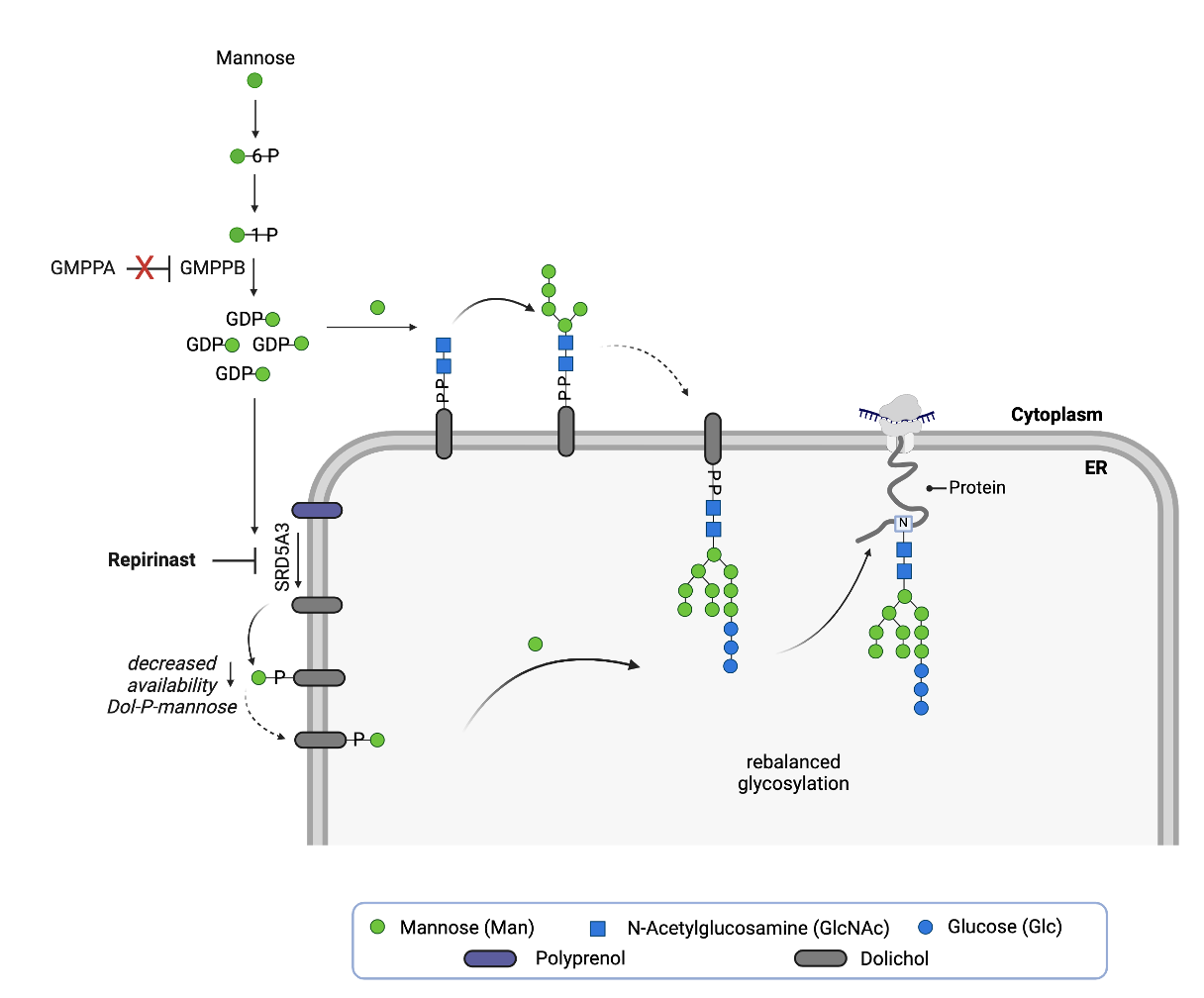
We believe the fly results below are telling us that the cell copes with GMPPA deficiency by reducing the availability of dolichol phosphate mannose (Dol-P-Man), the universal mannose donor for multiple steps in the N-linked glycosylation pathway. Less Dol-P-Man is available, which in the absence of a way to reduce GDP-mannose levels directly appears to be the safest and most effective way for the cell to deal with excess GDP-mannose it cannot for some reason easily and safely shunt or metabolize away.
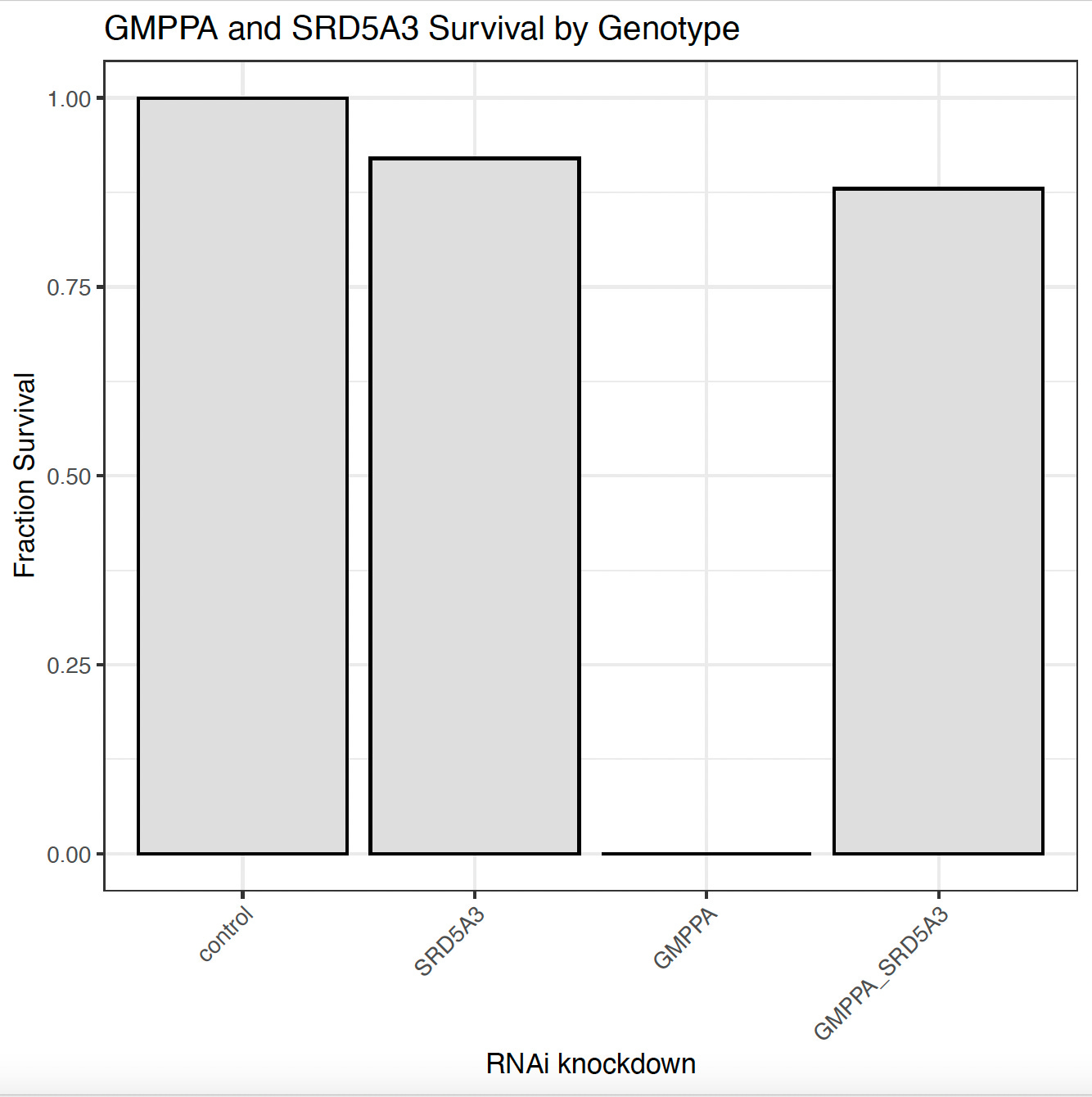
In the absence of molecular target data, the GMPPA-CDG fly model provides the most compelling evidence that repirinast is a cryptic SRD5A3 inhibitor. Knockdown of SRD5A3 alone is not lethal, but knockdown of GMPPA alone is. However, knockdown of both GMPPA and SRD5A3 results in near complete rescue of lethality. Fly genetics confirms the pharmacology and a “two wrongs make a right” mechanism.
We’ve seen the “two wrongs make a right” rescue mechanism before in CDG across multiple model systems, even in humans. In yeast, for example, where genetic inhibition of PGM1 rescues PMM2 deficiency. In flies, DPM1 is as a disease modifier of DPAGT1, just like in the GMPPA/SRD5A3 case. And in humans, ALG6 mutations modify PMM2-CDG disease severity.
The call is coming from inside the house!
If you think about it, where else could it be coming from? Like any small-world network, there are intimate and redundant connections between nodes in cellular metabolism. What’s not obvious is the disease modifier pairs among all CDG genes. Efforts should be made to map the CDG disease modifier network. What we can say for certain now is that the evidence for a linkage between GMPPA and SRD5A3 is based both on fly genetics and first-principles pharmacology.
Ironically, we found a different pharmacophore (mevalonate) enriched in SRD5A3 rescuers, but the rebalancing principle is the same. In SRD5A3 deficiency, upstream metabolic flux is reduced at the source by inhibiting mevalonate production by the enzyme HMG CoA. The amount of polyprenol flowing to SRD5A3 is reduced to match its lower catalytic capacity. Two wrongs make a right, genetically and pharmacologically, seems to be the order of the day.
You might be asking yourself: why not start a single-patient IND study for repirinast just like we did years ago for epalrestat, another Japanese drug repurposed for a CDG?
Ideally to buttress the fly data we’d like data from Rosie’s cells showing improvement in glycosylation upon treatment with repirinast. That would connect the critical dots between flies and human cells; and between SRD5A3 inhibition and rebalanced glycosylation at the proteome level. Those experiments were completed and analysis did not reveal a clear rescue signal. We’ve seen this movie before though. Fibroblasts are not neurons, and neurons are not brains. A fly does happen to combine all all three bit to some extent.
However, there was a practical blocker to a repirinast spIND study: drug manufacturing. Unlike in the case of epalrestat, which is still marketed in Japan and available by doctor’s order, repirinast was commercially discontinued years ago. This is the fate of many drugs around the world: supplanted by next-generation successors with improved efficacy or simply on-patent derivatives that have greater revenue potential. Bloom for a Cure has both an ODD and RPDD granted for repirinast and GMPPA-CDG. But manufacturing costs are in the hundreds of thousands of dollars. Not a trivial commitment to make without having more confidence that the drug will actually work in a human.
As a middle ground, Diana decided to pursue a spIND for finasteride, which is available by prescription in the US. The fly data tell us that finasteride is targeting SRD5A3, close cousin to its intended target SRD5A2. After getting buy-in from a pediatric endocrinologist and in consultation with a team that includes a local physician so as to minimize travel for site visits, a finasteride single-patient observational study has officially begun for GMPPA-CDG pioneer Rosie under the direction of Dr Eva Morava-Kozicz at Mount Sinai in New York City.
Rosie took her first dose of finasteride on Sunday July 27, 2025.
A protocol was devised that assesses safety and a suite of exploratory outcome measures. We’re focused on tests that could objectively read out efficacy. One extremely practical outcome measure happens to be in play for GMPPA. Namely, alacrima, or lack of tear production. Similar to NGLY1 deficiency, alacrima is a core symptom of GMPPA deficiency. It’s a rare symptom in CDGs overall, and how deep the linkage between GMPPA and NGLY1 goes is still the bleeding edge of science.
Harkening back to Matt Might showing his dearly departed son Bertrand’s first tears after a N-of-1 drug repurposing trial, alacrima could act as a rapid indicator of target engagement and therapeutic effect. It’s reminiscent of keratosis pilaris responding rapidly to ibuprofen treatment in MAN1B1-CDG. Swallowing would be the next area of improvement, as Rosie takes in a good chunk of her daily calories through liquid or puree. If she is able to swallow food, that would be a quantitative biomarker with real-world positive impact day to day. Cognitive and motor improvements would be the other areas to look for potential treatment effect. We don’t know which symptoms or disease processes will respond to treatment.
This is why we N-of-1.