We found PGAP3 yeast hits! Now what?
Results from drug repurposing screens using a yeast model of PGAP3 deficiency suggests cell death from ferroptosis as a pathomechanism, and phospholipase A2 activation as a therapeutic strategy.
In collaboration with
Disclaimer
The results of the PGAP3 drug repurposing project that we are sharing in the spirit of open science below are novel preclinical research findings and therefore they do not constitute the practice of medicine. Please consult your physician or clinical care team if you’re considering off-label use of any approved drug. The same caution applies to nutraceuticals, supplements and “generally recognized as safe” compounds.
Last time we shared that all PGAP3 yeast drug repurposing screens have been completed on behalf of Moonshots for Unicorns. In this followup post, we’ll explore the structure-activity relationships of the PGAP3 rescuer hits and offer therapeutic interpretations of the data. When interpreting phenotypic screening data, it’s best practice to rule out the most parsimonious model first — until it’s the last one standing.
We already established a convergence on ferroptosis in both the PGAP3-deficient yeast model and Lucy’s iPSC-derived astrocytes and neurons. Based on results from Professor Kathrin Meyer’s lab, it appears that we’re dealing with a “piano” — problem in astrocytes, not neurons — versus a “piñata” — problem in neurons, not astrocytes. Yeast being an undifferentiated single-cell organism means it could be modeling aspects of the astrocyte experience and the neuron experience at the same time. We’ll return to this point at the end.
But before we explore the Pharmakon and ReFRAME datasets in depth, let’s quickly review the known biology about PGAP3, which is short for Post-GPI-Attachment to Proteins 3. To do so, we have to zoom out on the entire GPI anchor biosynthetic pathway aka supply chain.
The PGAP3 CDG Hub page illustrates with both artistic and technical precision how the GPI anchor supply chain spans the secretory pathway, from ER to Golgi to vesicles bound for delivery of over 150 different GPI anchor proteins to the plasma membrane. One such GPI anchor protein is called ceruloplasmin, a copper-dependent enzyme that is part of the high-affinity iron uptake system that is conserved from yeast to humans.
Let’s zoom in on panel 4 to see exactly which step in the GPI anchor supply chain PGAP3 functions. As described in Fujita et al., 2006, the original data on PER1, the yeast version of PGAP3, raised the question if PER1 encodes a phospholipase A2 enzyme, or whether PER1 actually encodes a regulatory protein that activates phospholipase A2.
Surprisingly, 17 years later, this remains an open question today.
When the unsaturated fatty acid chain (depicted as the kinked dark gray line) cannot be clipped off by phospholipase A2 in time, a pathological cascade likely ensues resulting in system-wide imbalance between unsaturated and saturated fatty acids — and therefore lipid peroxidation by iron-induced reactive oxygen species aka a downward ferroptic spiral. The saturated fatty acid is waiting in vain for an opening that never arrives; the unsaturated fatty acid is not removed from the GPI anchor and so un-remodeled GPI anchor proteins are not properly delivered to the plasma membrane.
If PGAP3 is not a phospholipase A2 itself, it may be the regulatory subunit that activates a ER or Golgi resident phospholipase A2. In that case, complete absence of PGAP3 would also result in loss of phospholipase A2 activity. Naturally, a small molecule that somehow pharmacologically substituted for PGAP3 would activate the resident phospholipase A2 instead.
In the absence of PGAP3, the nascent GPI anchor would be destined to be sub-functional or nonfunctional, triggering secondary stress response cascades. Specific GPI anchor proteins would fall into deficit, further inflaming the situation, depending on which cell type is most vulnerable to the loss of that particular GPI anchor protein.
Only one way to find out..
Previously, we presented a high-level summary of the ReFRAME library screen. Before that, we first shared top-line results from the Pharmakon library screen. We’ll focus exclusively on the rescuer hits for now and what they tell us about therapeutic strategies that either help cells cope with ferroptotic stress or target the root cause of disease by pharmacologically compensating for the missing PGAP3 function.
The top 20 Pharmakon rescuers are enumerated in the plot below. All 20 rescuers have Z-scores greater than 2.5 standard deviations above the mean. After we first communicated these results to the Landmans six months ago, they started giving Lucy three OTC nutritional supplements marked in bold: #4, #7, and #11.
Mifepristone was removed from consideration because it is a so-called pan assay interference compound. That’s a longwinded way of saying it’s a false positive. It was also a singleton in terms of structure and pharmacological class. One must be wary of false positives in any screen and either have a counter-screen in the hit validation stage or be able to surmise that a compound class is false positive for the specific assay used in the screen.
The three most immediately clinically actionable hits are:
chlorophyllide, a chlorophyll analog that coordinates a copper ion at its center
folic acid, a derivative of vitamin B and naturally occurring antioxidant
alpha lipoic acid, a naturally occurring antioxidant
Based on first principles, these compounds are not addressing the root cause of PGAP3 deficiency. Instead, the best they could do therapeutically is to help vulnerable cells cope with the consequences of missing PGAP3, namely iron-mediated or ferroptotic stress. Meanwhile, inflammation smolders unchecked at its source.
Chlorophyllide generated sparkles of efficacy in Lucy but it was not sustainable due to poor bioavailability. It does turn her poop a delightful forest green, Geri commented in passing in an email last October. Lucy remains on folic acid and alpha lipoic acid to this day. She was taken off tyrosine due to sleep side effects. I had to stop the tyrosine because it was interfering with her sleep and she started waking up 3-5 times per night, which wasn't sustainable for anyone, Geri explained in another email last December.
Here are the 20 PGAP3 yeast rescuers organized by presumed pharmacological class, with structural analogs grouped together.
The orange box contains cationic amphiphilic drugs that act as local anesthetics. The purple box contains lipophilic antioxidant vitamins, two of which — folic acid and alpha-lipoic acid — are already part of OTC mitochondrial cocktails. Here’s a paper showing that folinic acid, a derivative of folic acid, had efficacy in a child with PGAP2 deficiency. The blue box contains chlorophyllide which acts as a copper carrier. The red box contains deferoxamine, an iron chelator. The green box contains antivirals, including a pair of structural analogs.
The gray box contains singleton hits. Note that sulbentine and allyl isiothiocyanate form a pharmacophore pair: the latter constitutes an “endopharmacophore” within the former. There’s actually a second example in dataset where a hit is structurally embedded inside another hit. Can you spot it?
None of the compounds in the green or gray boxes have an obvious mechanism of action that would be evolutionarily conserved in both yeast cells and Lucy’s astrocytes and neurons. The iron chelator deferoxamine is FDA approved for acute iron poisoning but the threshold for use is higher, especially as the target tissue is the brain. With the blue and purple boxes tapped out, that left the orange box. Local anesthetics and acute blood pressure medications are not nearly as inviting as the OTC supplements.
However, the structure-activity relationship is striking in the local anesthetics cluster. Of course, they’re also CADs: cationic amphiphilic drugs. Isobutamben and butamben isomers, i.e., they have the same exact number of atoms but isobutamben contains a 2-methylpropyl ester side chain while butamben has a butyl side chain. Interestingly, the fourteen other local anesthetics in the Pharmakon library, including procaine shown below, were inactive.
Could these drugs be activating phospholipase A2 activity in the absence of PGAP3?
Okay, here’s the moment you’ve all been patiently waiting for: a comparison of rescuers from the Pharmakon library screen versus the ReFRAME library screen.
5 out of the 6 tops hits with the highest Z-scores in the ReFRAME dataset are….ATP analogs, including ATP itself. And remdesivir. Remember that drug from the start of the COVID pandemic? Although the top hits weren’t ivermectin or its analogs, the pattern immediately sounded alarm bells.
None of the five ATP-containing compounds were present in the Pharmakon library and we didn’t know beforehand that they would be in the ReFRAME library. The cationic amphiphilic in vitro tool compound phortress is the only top Z-scoring hit that doesn’t appear to be an artifact of using a lucerifase-based luminescence assay where exogenous ATP directly activates luciferase.
An examination of the shape of dose response curves is consistent with the conclusion that ATP, remdesivir and diadenosine tetraphosphophate are all false positives. The nail in the coffin is the inactivity of the non-hydrolyzable version of ATP that lacks the high-energy phosphoanhydride bond to the terminal phosphate.
Having set aside the ReFRAME false positives, let’s take a closer look at the ReFRAME true positives. The most potent hit from the entire ReFRAME library screen is lercanidipine, a calcium channel blocker approved for hypertension. Remarkably, lercanidipine is also a Pharmakon rescuer! Lercanidipine is the only compound that rescued in both Pharmakon and ReFRAME screens.
Because there is no yeast version of the targets of JTV-803, vorapaxar, histrelin, cobimetinib, seviteronel or SQ-10996 (a carbamazapine analog from the 1970s), we don’t understand their mechanism of action in the context of PGAP3 deficiency in yeast. At this point we can only speculate, which is why it’s essential to perform confirmatory experiments in other PGAP3 disease models before diving down a pharmacological rabbit hole.
Here are the biphasic hits, which rescue growth at lower doses up to a maximum dose, after which the compounds become toxic.
Note that phortress and phytosphingosine are both cationic amphiphiles: a positively charged amino group on one end, and a lipophilic aromatic system or acyl chain on the other end. HX-1171 is a lipophilic vitamin E derivative, which the reader will appreciate echoes the results from Pharmakon.
Perhaps the most intriguing hit is goxalapladib, which was developed as a phospholipase A2 (PLA2) inhibitor. You can see the letters '“pla” embedded in the name. The dose response data are telling us that at sub-lethal doses, goxalapladib may be acting as a PLA2 activator. This type of biphasic behavior — low dose = rescue; high dose = sensitization — is not unusual in pharmacology.
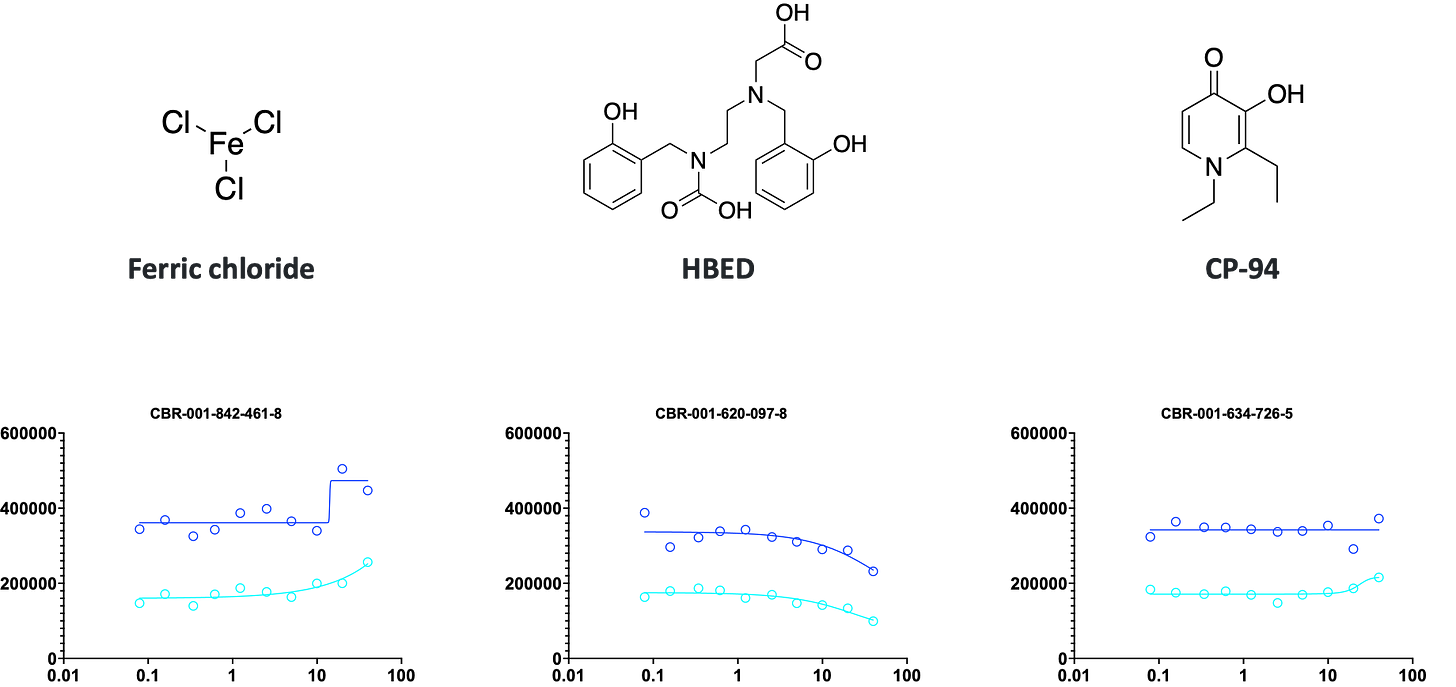
What about the iron and copper clusters from Pharmakon? Do they emerge from the ReFRAME too? The answer is, yes but with an asterisk.
Interestingly, but also a bit paradoxically, we see divergent results between Pharmakon vs ReFRAME, and within ReFRAME. In the iron cluster, we have two iron chelators — HBED and CP-94, the latter being a derivative of deferiprone — and a source of iron itself in the form of ferric chloride. One of the iron chelators, HBED, appears to have a weak sensitizing effect, while the other iron chelator, CP-94, appears to have a weak rescuing effect. Ferric chloride also appears to effect a mild rescue.
We see the same paradoxical behavior in the copper cluster. CuATSM, a synthetic copper carrier, and copper disodium EDTA both have sensitizing effects. The opposite of what we expected based on the Pharmakon experience with chlorophyllide.
Remarkably, we observed exactly that pattern of opposite activities of iron chelators in the results of the PIGA Pharmakon screen. Take-home message: not all iron chelators or copper carriers are equal! Why might this be, setting aside differences in the pharmacokinetics and pharmacodynamics of each iron chelator or copper carrier?
As alluded to at the start of this update, a yeast cell is a single-cell organism. Therefore a yeast cell is sort of like having Lucy’s astrocytes and neurons — and every other differentiated cell lineage — crammed into a single representative cell whose only imperative is to grow. Neurons are famously post-mitotic and completely dependent on astrocytes and other glial “support” cells for nutrients and waste removal, including iron and copper balance. The physiological state of a yeast cell may approximate that of an astrocyte in one context, but that of a neuron in another context.
Finally, we present a summary slide of the ReFRAME library rescuer hits.
Although the members of each of the hit classes are different, we nonetheless still see the same five classes emerge from both Pharmakon and ReFRAME screens:
membrane-active cationic amphiphilic drugs
lipophilic antioxidant vitamins
iron chelators
copper carriers
L-type calcium channel blocker
What’s next?
More hit validation studies are needed to test the phospholipase A2 activation hypothesis. Phytosphingosine should be prioritized since it’s an OTC supplement, though not as commonly used as the aforementioned lipophilic antioxidant vitamins.
More hit validations studies are needed to examine iron and copper balance and to parse out cell autonomous effects — piñata: problem in neurons, not astrocytes — versus cell non-autonomous effect — piano: problem in astrocytes, not neurons.
Definitely more hit validation studies are in order to test lercanidipine, which emerged as the wildcard rescuer. The other singleton hits can’t be entirely dismissed but right now not there aren’t enough threads to pull on to be informative versus speculative, which can be dangerous.
The yeast PGAP3 deficiency model has done its part. Now it’s time for Lucy’s iPSC-derived astrocyte and neuron models to step up to the plate. We propose measuring GPI anchor lipid remodeling in response to drug treatments directly in Lucy’s cells. In parallel, iron and copper biomarker discovery studies should commence in PGAP3 kiddos, starting with Lucy. And it goes without saying that worm or fly or zebrafish models of PGAP3 deficiency would have enormous utility, too.
Onward for Lucy and all the other PGAP3 kiddos!