Yeast-powered drug repurposing screen ➡️ parent-driven N-of-1 study
In collaboration with a pioneer PIGW-CDG family, we identified two over-the-counter nutraceuticals as actionable hits from a yeast screen. The combination treatment is working.
In collaboration with
Disclaimer
The results of the PIGW-CDG drug repurposing project that we are sharing in the spirit of open science below are novel preclinical research findings and therefore they do not constitute the practice of medicine. Please consult a physician or clinical care team if considering off-label use of any approved drug or compassionate use of any experimental drug. The same caution applies to nutraceuticals, supplements and “generally recognized as safe” compounds.
A scattering of PIGW-CDG patients is known to medicine today, and young Hannes is one of them. His parents, Kelsey and Brian, had to take matters into their own hands because no TradBio white knight was coming to their rescue. That meant sticking their necks out — and stretching their wallets thin — to pay for research that had never been done before. It’s what pioneer families do. We’ve seen it over and over again at Perlara, settling any question about repeatability.
One of the clutch learnings of the last two years of offering yeast-powered “drug repurposing as a service” is that inherited metabolic diseases, especially CDGs, are where yeast patient avatars shine the brightest. To date, we have zig-zagged across the GPI (glycosylphosphatidylinositol) anchor biosynthesis landscape, hopping from enzyme deficiency to enzyme deficiency purely based on which pioneer families show up on our doorstep.
Whether by chance or providence, we started with biosynthetic bookends: the GPI-GnT complex member PIGA, at the start of the supply chain; and the GPI-TA complex member PIGS, which attaches a protein to a GPI anchor that later goes onto post-production editing in the secretory pathway. Following the lead of pioneer families, we also sampled the middle of the GPI supply chain: the PIGN enzyme, which plops the first phosphoethanolamine moiety onto the nascent GPI anchor.
The PIGW enzyme is sandwiched between PIGA and PIGN, where the GPI anchor assembly flips from the cytoplasmic face of the ER to the lumen of the ER. This step in the pathway depends on fatty acid availability because PIGW is an inositol acyltransferase. Its job is to attach a specific type of fatty acid to the inositol ring of the GPI anchor.

The resulting three-tailed GPI anchor intermediate exists while the GPI anchor is built up and the protein is appended. After the protein and GPI anchor are covalently linked, the lipid tails undergo a multi-step removal and remodeling process. PGAP1, the alter ego of PIGW, is an inositol deacylase. It clips the acyl tail that was added by PIGW.
Why does nature attach a third acyl chain to the growing GPI anchor only to later shear it off? Our intuition around the intersection of lipid and sugar supply chains is defined by our experience with PGAP3-CDG, the first instance where we went straight from yeast drug repurposing hits to a parent-driven n-of-1 study in the fall of 2022.
The antioxidant PGAP3-CDG rescuers like alpha-lipoic acid and folic acid that are standard components of mitochondrial cocktails were a clue. In parallel, it just so happened that disease modeling experiments on patient-derived iPSC derived astrocytes were being conducted in Dr Kat Meyer’s lab at Nationwide Children’s Hospital. Remarkably, Lucy’s astrocytes and the PGAP3-deficient yeast avatar were both experiencing a lipid peroxidation crisis aka ferroptosis.
PGAP3 is a phospholipase that removes an acyl chain while PIGW is an acyltransferase that adds an acyl chain. Even though these enzymes catalyze opposing reactions, maybe they both can cause a lipid traffic jam? In the same way a revolving door can get stuck in either the clockwise or the counter-clockwise rotation.
One more item to note about PGAP3-CDG. In the case of PGAP3-CDG, Lucy’s parents are both medical doctors. Another pair of M.D. parents whose son Jake has MAN1B1-CDG were also drug repurposing first movers and perhaps willing to take on more risk than a doctor not personally affected, let alone pioneer parents who are not doctors. Now imagine that one parent was trained as an architect and the other as a software engineer, neither with scientific or medical credentials. Can they still drive clinical research forward?
When a disease is as tiny as PIGW-CDG, the answer is a resounding yes.
In the previous installment of the PIGW-CDG Cure Odyssey, we were about to perform the TargetMol library screen. The first attempt last August yielded a noisy (unusable) dataset, so we had to repeat the screen. The second bite at the apple a month later proved successful.
Small molecules don’t have personalities in the conventional sense but they are nonetheless colorful. Here’s a representative plate from the TargetMol library. Note that part of the reason we use a luminescence-based readout is so as not to have issues with false positives due to overlapping emission spectra, which affect fluorescence-based and absorbance-based measures of yeast cell growth.
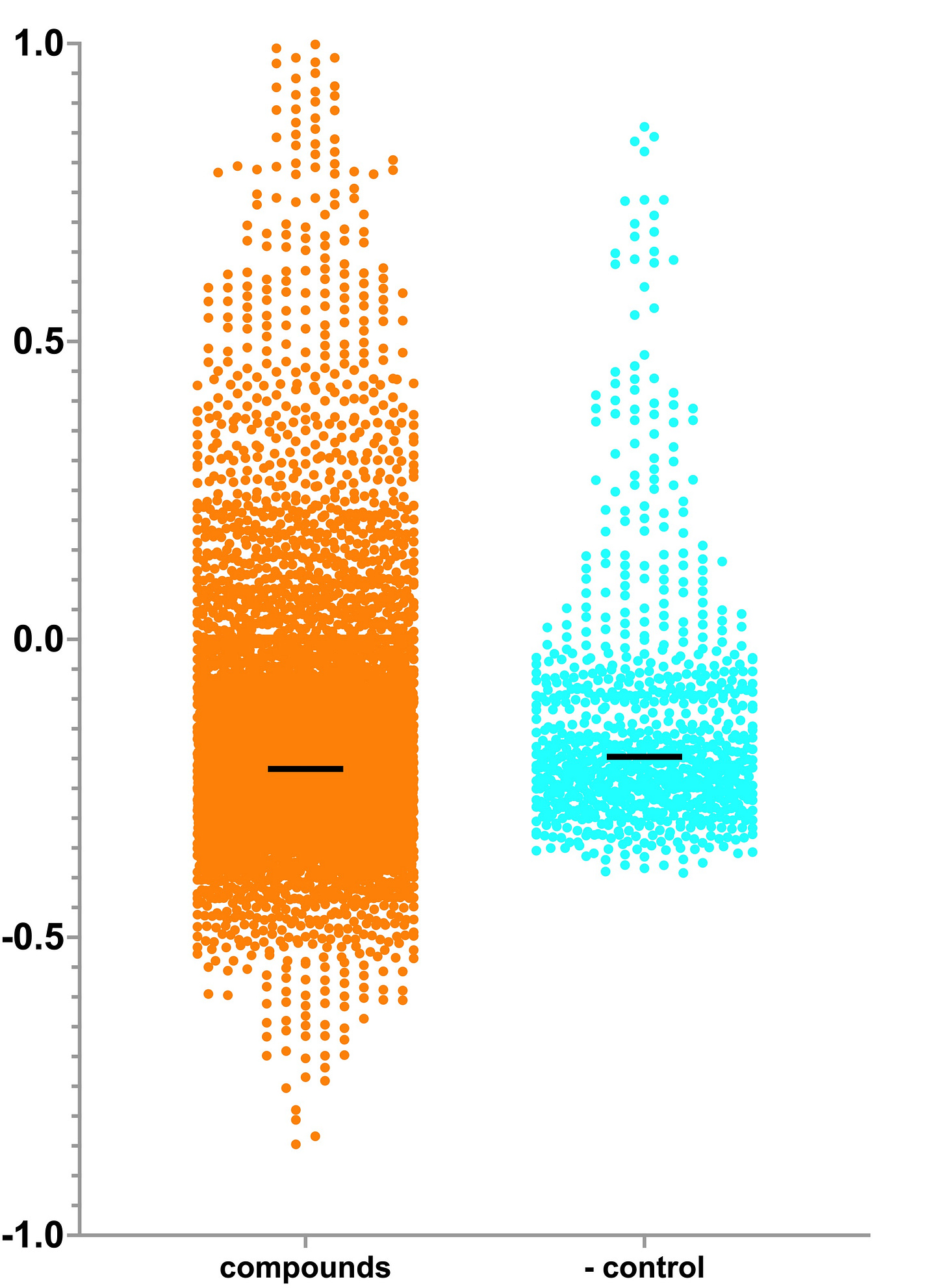
Let’s start the TargetMol screen analysis with the Z-score distribution. For ease of viewing, the y-axis is segmented. The positive control is a wild-type (healthy) yeast strain. The negative control is the temperature-sensitive PIGW yeast avatar.
There are 35 presumptive rescuers with a Z-score greater than 1. The grey circle is ADP, which is a positive control that tells us the screen was performant and had a dynamic range comparble to previous TargetMol screens. That takes us down to 34 presumptive rescuers. One of those is what we refer to as a jackpot: a runaway screening hit that exceeds the positive controls. Like elsewhere in life, it turned out to be too good to be true. We’ll return to Compound 1 and Compound 2 in a moment.
First, let’s zoom in closer on the presumptive rescuers and presumptive sensitizers:
There’s an enrichment of presumptive rescuers in the Z-score range 0.5 to 1 even though the distributions are overlapping. The PIGW temperature-sensitive yeast avatar is on the leakier side, meaning sometimes the mutant grew better than expected. Something about the intrinsic biology makes the growth defect not fully penetrant. Interestingly, the PGAP3-deficient yeast avatar was also leaky.
On the other hand, there’s an obvious and substantial enrichment of presumptive sensitizers with a Z-score lower than -0.5. We never observed any of the negative control wells with a Z-score below -0.5. We observed this skewed ratio of sensitizers to rescuers in PIGA-CDG, and to lesser degrees in ALG11-CDG and PMM2-CDG. However, PIGN-CDG and PIGS-CDG screens did not yield an excess of sensitizers. If anything, the sensitizer-to-rescuer ratio is skewed in the opposite direction. Based on available data, there doesn’t seem to be any rhyme or reason to which way that ratio skews. But as we add more nodes to the map, we’re confident that an explanation will emerge.
In the meantime, we can start to compare and contrast different CDG TargetMol screens. For example, here’s a Z-score scatterplot with PIGW-CDG results on the x-axis and PMM2-CDG result on the y-axis. The only common rescuer is the positive control ADP, which serves as a sanity check that the screens worked. On the other hand, there is a trend toward convergent sensitizers.
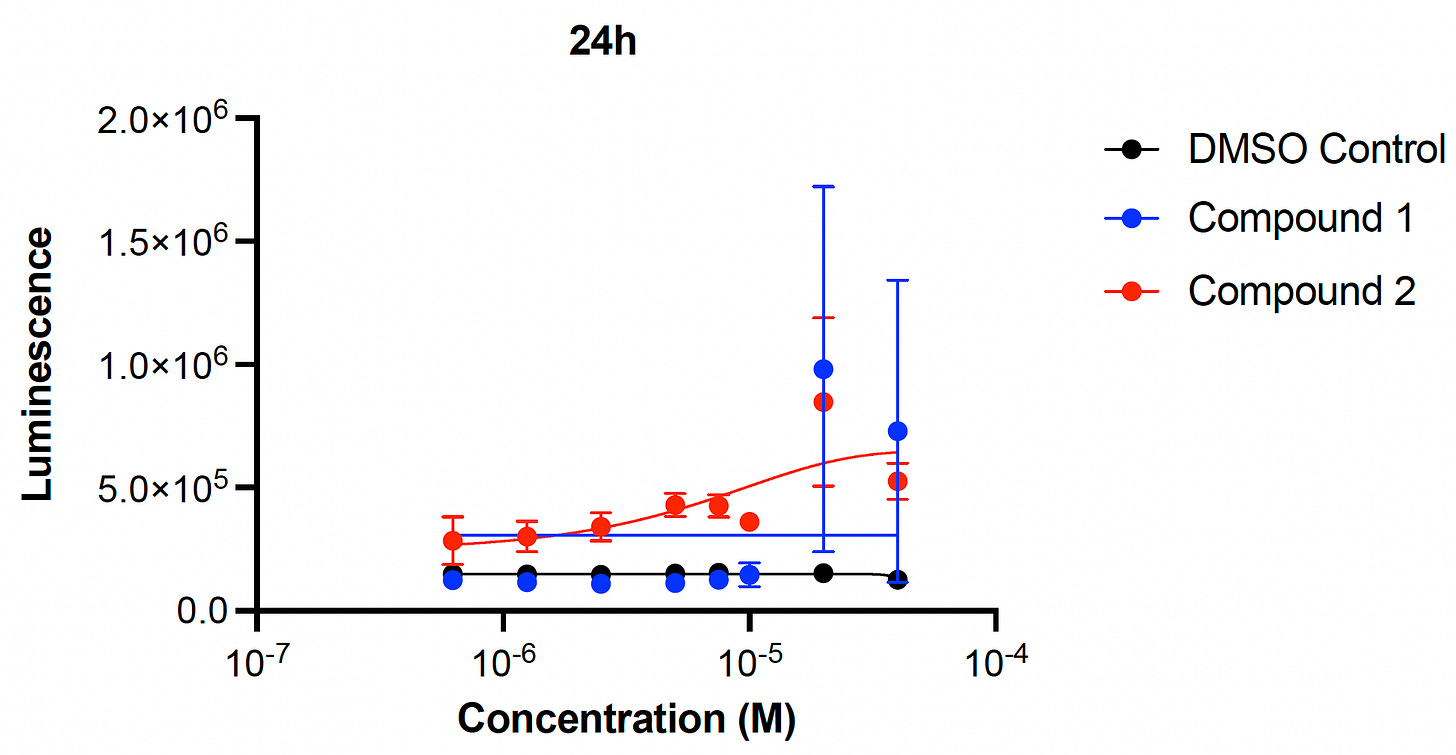
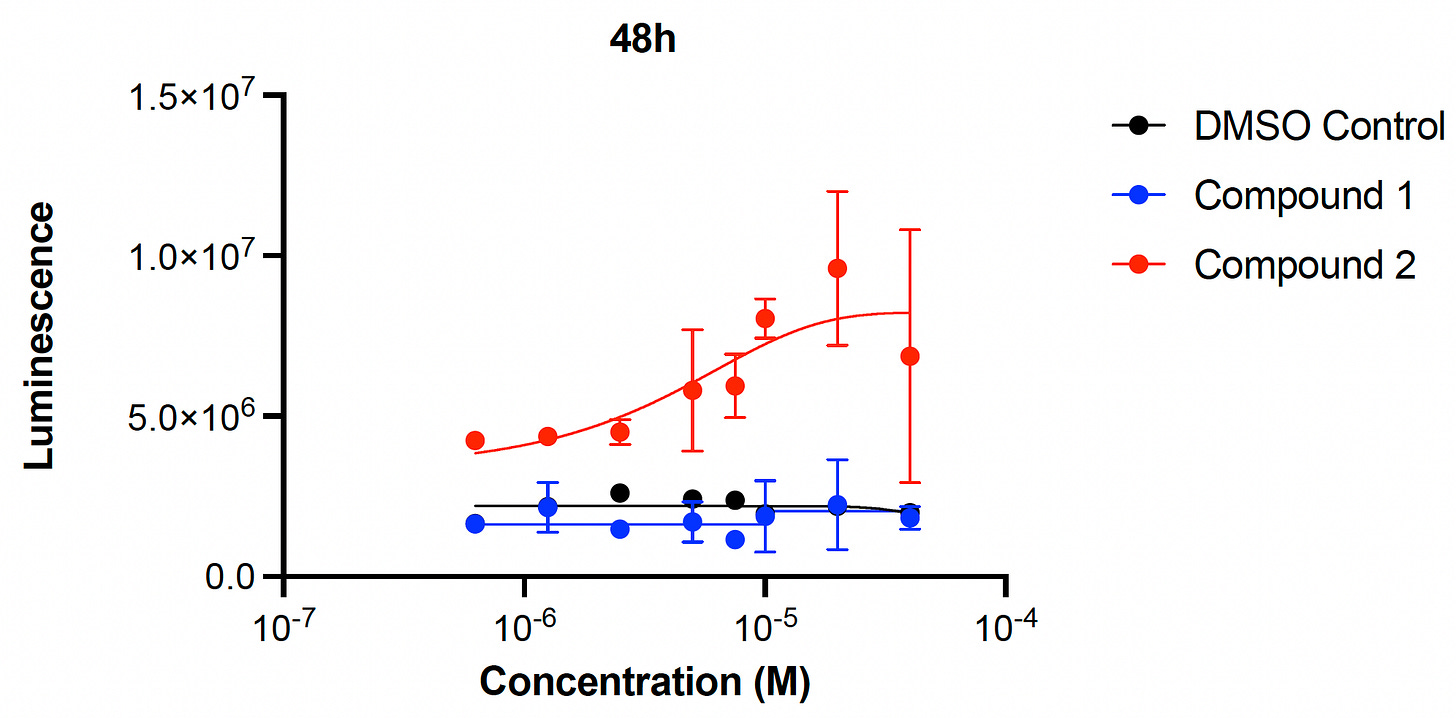
Okay, remember presumptive rescuer Compound 1 and presumptive rescuer Compound 2? We focused on these two presumptive PIGW-CDG rescuers because they are both over-the-counter nutraceutical supplements available for purchase online from multiple reputable manufacturers. We first performed 8-point dose response experiments on Compound 1 and Compound 2 individually and measured yeast cell growth after 24 hours and 48 hours of compound treatment.
Compound 1 rescues, albeit with high variance, at the highest two doses at 24 hours, but not at 48 hours. On the other hand, Compound 2 exhibits robust rescue at both 24 hours and 48 hours, and also appears to be more potent than Compound 1.
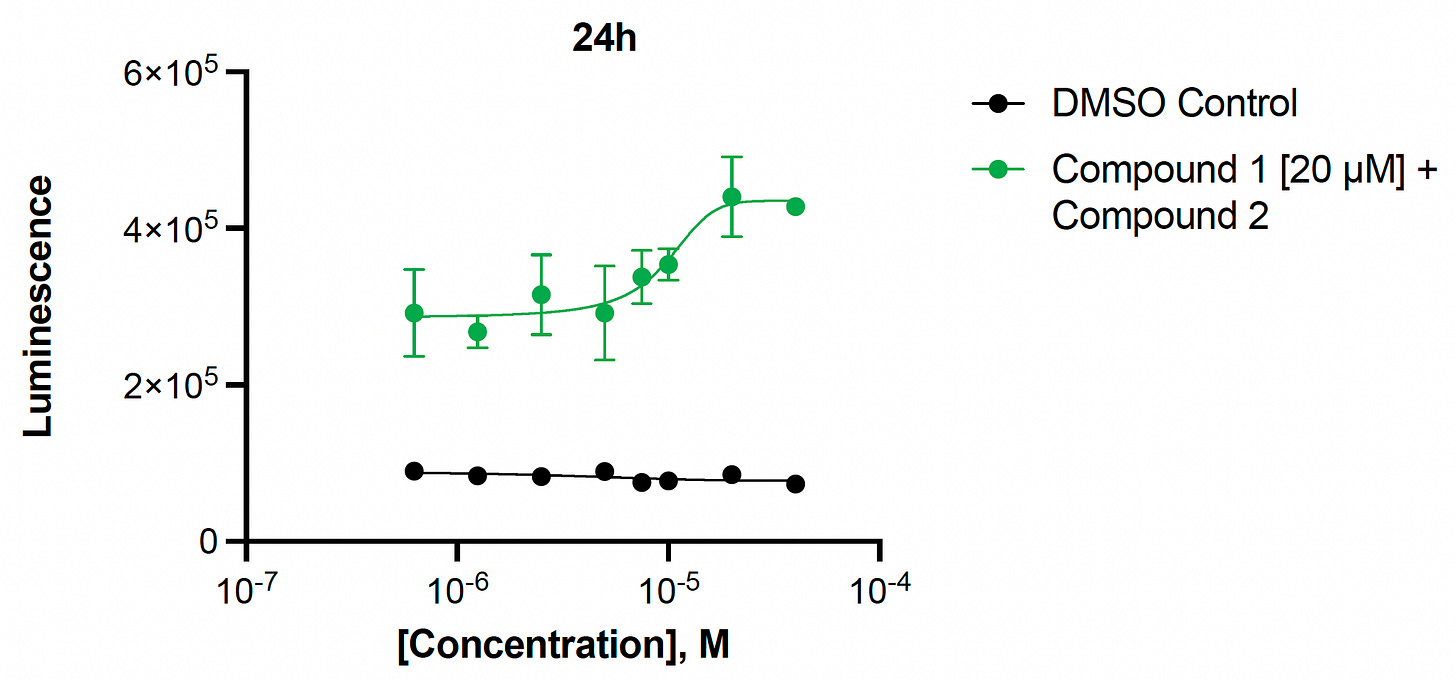
Next, we performed a Compound 1 + Compound 2 combination experiment. In contrast to ALG11-CDG which has a ceiling to the rescue effect, the absolute rescue effect of the combination treatment increases from 24 hours to 48 hours. The magnitude of the rescue is quite large, suggesting that the primary cellular pathophysiology is being rescued.
Buoyed by those encouraging hit validation experiments using the PIGW-CDG yeast avatar, Kelsey and Brian put the presumptive rescuer hits to the ultimate test: their son Hannes. Using video capture by iPhone as a digital outcome measure, Kelsey performed baseline assessments of gross motor function, to use clinical trial parlance. Here’s a video taken in the lead-up to administration of Compound 1. Hannes is unable to stand on his own and required physical support to maintain an upright posture.
Here’s another baseline video of Hannes a few days before Compound 1 treatment began last fall.
Kelsey supplemented objective videos with real-world accounts of Hannes’ reaction to treatment with Compound 1. Despite being a parent-led n-of-1 study, Kelsey and Brian enforced pseudo-blinding by not telling anyone else in Hannes’ orbit about what was going on. That means other caretakers and family members in Hannes’ life were unaware that he was on any intervention. So, if those blinded individuals independently observed and reported similar changes in Hannes, it would soften concerns that Kelsey and Brian are biased observers.
And I can definitely fill you in on Hannes' progress recently, but our overarching opinion is that for the first couple weeks we didn't see much difference and then in the last 2 weeks we feel like there was a change. He seems to be a lot more actively aware rather than passively aware. The best way to describe it is that it seems like he's processing more quickly and purposefully (like if there's a toy that you put down that he wants, he makes the decision almost instantaneously to pivot and move to it). He's choosing to move to where his brothers are instead of just being excited when they come to him, and he's more purposeful in his movements (like trying to pick up everything on a table in front of him rather than just what's immediately in front of him). Interestingly, I think we're noticing more cognitive changes than physical, but that might just be because he's been on a more consistent physical development path (definitely behind typical development, but continuously developing) whereas the cognitive piece has been harder to see consistent development or jumps in development until now.
Three weeks later, Kelsey emailed this dispatch from the n-of-1 frontlines:
Otherwise, we're continuing to see awareness way beyond anything he was doing before we started the supplement (and I think we may be seeing some improvement in his physical strength now that's faster than what we'd seen before too). For example, I feel like he's playing with his brothers differently which is coming through in terms of his awareness/decision making/social back and forth and the speed he's able to physically do things to engage more with them (for example, his 4 year old brother crawled away from him to try to get him to chase, and he immediately crawled after him, which I've never seen him do before).
Here’s the video receipt:
After several months on Compound 1 alone, Kelsey and Brian added Compound 2 to the stack. Hannes continued to show gains and improvements, as demonstrated by this video of Hannes on a balancing board. Look out, Tony Hawk! You’ve got competition now.
Can we ascribe with 100% certainty Hannes’ progress to Compound 1 and Compound 2 combination treatment? No. We don’t know how Hannes would have progressed over these past 6-9 months if he hadn’t been on any interventions. The only way to find out is to go from N-of-1 to 1-to-N. If a second and third PIGW-CDG pioneer respond similarly to the Compound 1 + Compound 2 combination, the case in favor of a treatment effect grows stronger.
Moves are afoot. Stay tuned for the next installment of the PIGW-CDG Cure Odyssey.












This is wonderful news about Hannes and it is so rewarding for me to be a part of this successful real-life patient story. It reminds me of my days as a postdoc funded by the PNW Mito Guild to work on Leigh syndrome using a worm model system, and I got the opportunity to meet patients impacted by this rare, debilitating disease. It's really a dream come true to contribute to Perlara's wonderful work.